Key Points
-
The approved dose of anticancer drugs is often derived from empirical, small-scale studies rather than rationally conducted trials incorporating appropriate a priori assumptions
-
The pharmacokinetic profiles of an individual taking anticancer drugs are assumed to be associated with anti-tumour effects and toxicity patterns
-
Intra-individual variation in pharmacokinetics can be accounted for by patient-specific variables and environmental factors, but inter-individual variation in pharmacokinetics is also affected by pharmacogenetic factors
-
To personalize anticancer treatment, molecular characteristics of the tumour are important as well as information on environmental factors and germline genetic variability to achieve individualized drug dosing
Abstract
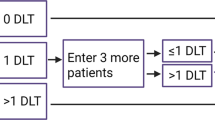
Identification of the optimal dose remains a key challenge in drug development. For cytotoxic drugs, the standard approach is based on identifying the maximum tolerated dose (MTD) in phase I trials and incorporating this to subsequent trials. However, this strategy does not take into account important aspects of clinical pharmacology. For targeted agents, the dose-effect relationships from preclinical studies are less obvious, and it is important to change the way these agents are developed to avoid recommending drug doses for different populations without evidence of differential antitumour effects in different diseases. The use of expanded cohorts in phase I trials to better define MTD and refine dose optimization should be further explored together with a focus on efficacy rather than toxicity-based predictions. Another key consideration in dose optimization is related to interindividual pharmacokinetic variability. High variability in intra-individual pharmacokinetics has been observed for many orally-administered drugs, especially those with low bioavailability, which might complicate identification of dose–effect relationships. End-organ dysfunction, interactions with other prescription drugs, herbal supplements, adherence, and food intake can influence pharmacokinetics. It is important these variables are identified during early clinical trials and considered in the development of further phase II and subsequent large-scale phase III studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mathijssen, R. H. et al. Flat-fixed dosing versus body surface area based dosing of anti-cancer drugs in adults: does it make a difference? Oncologist 12, 913–923 (2007).
Moore, M. J. Clinical pharmacokinetics of cyclophosphamide. Clin. Pharmacokinet. 20, 194–208 (1991).
Hryniuk, W. M., Figueredo, A. & Goodyear M. Applications of dose intensity to problems in chemotherapy of breast and colorectal cancer. Semin. Oncol. 14 (Suppl. 4), 3–11 (1987).
Tannock, I. F. et al. A randomized trial of two dose levels of cyclophosphamide, methotrexate, and fluorouracil chemotherapy for patients with metastatic breast cancer. J. Clin. Oncol. 6, 1377–1387 (1988).
Dent, S. F. & Eisenhauer, E. A. Phase I trial design: are new methodologies being put into practice? Ann. Oncol. 7, 561–566 (1996).
Rini, B. I. et al. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J. Natl Cancer Inst. 99, 81–83 (2007).
Schmidinger, M. et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 26, 5204–5212 (2008).
Motzer, R. J. et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N. Engl. J. Med. 369, 722–731 (2013).
Smith, D. B. et al. A phase I and pharmacokinetic study of amphethinile. Br. J. Cancer 57, 623–627 (1988).
Penel, N. & Kramar, A. What does a modified-Fibonacci dose-escalation actually correspond to? BMC Med. Res. Methodol. 12, 103 (2012).
Chabner, B. A. & Longo, D. L. (Eds) Cancer Chemotherapy and Biotherapy: Principles and Practice, 5th edn (Lippincott Williams and Wilkins, 2011).
Weiner, G. J. Monoclonal antibody mechanisms of action in cancer. Immunol. Res. 39, 271–278 (2007).
Parulekar, W. R. & Eisenhauer, E. A. Phase I trial design for solid tumor studies of targeted, non-cytotoxic agents: theory and practice. J. Natl Cancer Inst. 96, 990–997 (2004).
Le Tourneau, C., Lee, J. J. & Siu, L. L. Dose escalation methods in phase I clinical trials. J. Natl Cancer Inst. 101, 708–720 (2009).
Kitzen, J. J., Verweij, J., Wiemer, E. A. & Loos, W. J. The relevance of microdialysis for clinical oncology. Curr. Clin. Pharmacol. 1, 255–263 (2006).
Goulart, B. H. et al. Trends in the use and role of biomarkers in phase I oncology trials. Clin. Cancer Res. 13, 6719–6726 (2007).
Adjei, A. What is the right dose? The elusive optimal biological dose in phase I clinical trials. J. Clin. Oncol. 24, 4054–4055 (2006).
Bergsland, E. & Dickler, M. N. Maximizing the potential of bevacizumab in cancer treatment. Oncologist 9 (Suppl. 1), 36–42 (2004).
Perez-Soler, R. The role of erlotinib (Tarceva, OSI 774) in the treatment of non-small cell lung cancer. Clin. Cancer Res. 10, 4238s–4240s (2004).
Mita, A. C. et al. Erlotinib 'dosing-to-rash': a phase II intrapatient dose escalation and pharmacologic study of erlotinib in previously treated advanced non-small lung cancer. Br. J. Cancer 105, 938–944 (2011).
Yeo, W. L. et al. Erlotinib at a dose of 25 mg daily for non-small cell lung cancers with EGFR mutations. J. Thorac. Oncol. 5, 1048–1053 (2010).
Binder, D. et al. Erlotinib in patients with advanced non-small-cell lung cancer: impact of dose reductions and a novel surrogate marker. Med. Oncol. 29, 193–198 (2012).
Van Oosterom, A. T. et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 358, 1421–1423 (2001).
Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J. Clin. Oncol. 28, 1247–1253 (2010).
Verweij, J. et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 364, 1127–1134 (2004).
Blanke, C. D. et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J. Clin. Oncol. 26, 626–632 (2008).
Sleijfer, S., Wiemer, E. & Verweij, J. Drug Insight: gastrointestinal stromal tumors (GIST)--the solid tumor model for cancer-specific treatment. Nat. Clin. Pract. Oncol. 5, 102–111 (2008).
Meric-Bernstam, F., Farhangfar, C., Mendelsohn, J. & Mills, G. B. Building a personalized medicine infrastructure at a major cancer center. J. Clin. Oncol. 31, 1849–1857 (2013).
Roychowdhury, S. & Chinnaiyan A. M. Advancing precision medicine for prostate cancer through genomics. J. Clin. Oncol. 31, 1866–1873 (2013).
Druker, B. J. et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 344, 1038–1042 (2001).
Demetri, G. D. et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 347, 472–480 (2002).
Talpaz, M. et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood 99, 1928–1937 (2002).
Sawyers, C. L. et al. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood 99, 3530–3539 (2002).
Kantarjian, H. M. et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood 101, 473–475 (2003).
Jabbour, E. et al. Imatinib mesylate dose escalation is associated with durable responses in patients with chronic myeloid leukemia after cytogenetic failure on standard-dose imatinib therapy. Blood 113, 2154–2160 (2009).
Leyland-Jones, B. et al. Dose scheduling—Herceptin®. Oncology 61 (Suppl. 2), 31–36 (2001).
Baselga, J. Phase I and II clinical trials of trastuzumab. Ann. Oncol. 12 (Suppl. 1), S49–S55 (2001).
Tokuda, Y. et al. Dose escalation and pharmacokinetic study of a humanized anti-HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. Br. J. Cancer 81, 1419–1425 (1999).
Drugs.com. Trastuzumab Dosage [online], (2014)
Tol, J. et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N. Engl. J. Med. 360, 563–572 (2009).
Rini, B. I. et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J. Clin. Oncol. 28, 2137–2143 (2010).
Gianni, L. et al. AVEREL: A randomized phase III trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J. Clin. Oncol. 31, 1719–1725 (2013).
Sandler, A. et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 355, 2542–2550 (2006).
Weber, J. S. et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J. Clin. Oncol. 26, 5950–5956 (2008).
Wolchok, J. D. et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 11, 155–164 (2010).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
US National Library of Medicine. Clinicaltrials.gov [online], (2014).
Sleijfer, S. & Verweij, J. The price of success: cost-effectiveness of molecularly targeted agents. Clin. Pharmacol. Ther. 85, 136–138 (2009).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
US National Library of Medicine. Clinicaltrials.gov[online], (2014).
La-Beck, N. M. et al. Factors affecting the pharmacokinetics of pegylated liposomal doxorubicin in patients. Cancer Chemother. Pharmacol. 69, 43–50 (2012).
Sparreboom, A. et al. Evaluation of alternate size descriptors for dose calculation of anticancer drugs in the obese. J. Clin. Oncol. 25, 4707–4713 (2007).
Lu, J. F. et al. Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer. Clin. Pharmacol. Ther. 80, 135–145 (2006).
Evens, W. E. & Pratt, C. B. Effect of pleural effusion on high-dose methotrexate kinetics. Clin. Pharmacol. Ther. 23, 68–72 (1978).
Franke, R. M., Carducci, M. A., Rudek, M. A., Baker, S. D. & Sparreboom, A. Castration-dependent pharmacokinetics of docetaxel in patients with prostate cancer. J. Clin. Oncol. 28, 4562–4567 (2010).
Wheeler, H. E., Maitland, M. L., Dolan, M. E., Cox, N. J. & Ratain, M. J. Cancer pharmacogenomics: strategies and challenges. Nat. Rev. Genet. 14, 23–34 (2013).
Relling, M. V. et al. Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J. Natl Cancer Inst. 91, 2001–2008 (1999).
De Jong, F. A., de Jonge, M. J., Verweij, J. & Mathijssen, R. H. Role of pharmacogenetics in irinotecan therapy. Cancer Lett. 234, 90–106 (2006).
Yap, T. A. et al. Phase I trial of a selective c-MET inhibitor ARQ 197 incorporating proof of mechanism pharmacodynamics studies. J. Clin. Oncol. 29, 1271–1279 (2011).
Yamamoto, N. et al. The effect of CYP2C19 polymorphism on the safety, tolerability, and pharmacokinetics of tivantinib (ARQ 197): results from a phase I trial in advanced solid tumors. Ann. Oncol. 24, 1653–1659 (2013).
Tascilar, M., de Jong, F. A., Verweij, J. & Mathijssen, R. H. J. Complementary and alternative medicine during cancer treatment: Beyond innocence. Oncologist 11, 732–741 (2006).
Van Leeuwen, R. W. F., van Gelder, T., Mathijssen, R. H. J. & Jansman, F. G. A. Drug-drug interactions with tyrosine kinase inhibitors: a clinical perspective. Lancet Oncol. (in press).
Fujita, K. Food-drug interactions via human cytochrome P450 3A (CYP3A). Drug Metabol. Drug Interact. 20, 195–217 (2004).
Sparreboom, A. & Baker, S. D. Pharmacokinetic studies in early anticancer drug development in Principles of Anticancer Drug Development, Ch. 8 (eds Hidalgo, M., Garrett-Mayer, E., Clendeninn, N. J. & Eckhardt, S. G.), 189–214 (Humana Press/Springer Science, 2011).
Crews, K. R. et al. Altered irinotecan pharmacokinetics in pediatric high-grade glioma patients receiving enzyme-inducing anticonvulsant therapy. Clin. Cancer Res. 8, 2202–2209 (2002).
Relling, M. V. et al. Adverse effect of anticonvulsants on efficacy of chemotherapy for acute lymphoblastic leukaemia. Lancet 356, 285–290 (2000).
Budha, N. R. et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: Is pH-dependent solubility the Achilles heel of targeted therapy? Clin. Pharmacol. Ther. 92, 203–213 (2012).
Eley, T. et al. Phase I study of the effect of gastric acid pH modulators on the bioavailability of oral dasatinib in healthy subjects. J. Clin. Pharmacol. 49, 700–709 (2009).
Takahashi, N., Miura, M., Niioka, T. & Sawada, K. Influence of H2-receptor antagonists and proton pump inhibitors on dasatinib pharmacokinetics in Japanese leukemia patients. Cancer Chemother. Pharmacol. 69, 999–1004 (2012).
Egorin, M. J. et al. Effect of a proton pump inhibitor on the pharmacokinetics of imatinib. Br. J. Clin. Pharmacol. 68, 370–374 (2009).
Sparano, B. A. et al. Effect on antacid on imatinib absorption. Cancer Chemother. Pharmacol. 63, 525–528 (2009).
Yin, O. Q. et al. Effect of the proton pump inhibitor esomeprazole on the oral absorption and pharmacokinetics of nilotinib. J. Clin. Pharmacol. 50, 960–967 (2010).
Oostendorp, R. L., Beijnen, J. H. & Schellens, J. H. The biological and clinical role of drug transporters at the intestinal barrier. Cancer Treat. Rev. 35, 137–147 (2009).
Sparreboom, A. et al. Pharmacogenomics of ABC transporters and its role in cancer chemotherapy. Drug Resist. Updat. 6, 71–84 (2003).
Eechoute, K. et al. Drug transporters and imatinib treatment: implications for clinical practice. Clin. Cancer Res. 17, 406–415 (2011).
Ratain, M. J. Flushing oral oncology drugs down the toilet. J. Clin. Oncol. 29, 3958–3959 (2011).
Sharma, M. R. et al. Evaluation of food effect on pharmacokinetics of vismodegib in advanced solid tumor patients. Clin. Cancer Res. 19, 3059–3067 (2013).
Szmulewitz, R. Z. & Ratain, M. J. Playing Russian roulette with tyrosine kinase inhibitors. Clin. Pharmacol. Ther. 93, 242–244 (2013).
Dy, G. K. et al. Complementary and alternative medicine use by patients enrolled onto phase I clinical trials. J. Clin. Oncol. 22, 4810–4815 (2004).
Durr, D. et al. St John's Wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin. Pharmacol. Ther. 68, 598–604 (2000).
Perloff, M. D. et al. Saint John's wort: an in vitro analysis of P-glycoprotein induction due to extended exposure. Br. J. Pharmacol. 134, 1601–1608 (2001).
Sparreboom, A. et al. Herbal remedies in the United States: potential adverse interactions with anticancer agents. J. Clin. Oncol. 22, 2489–2503 (2004).
De Jong, F. A. et al. Lifestyle habits as a contributor to anti-cancer treatment failure. Eur. J. Cancer 44, 374–382 (2008).
Mathijssen, R. H. et al. Effects of St John's wort on irinotecan metabolism. J. Natl Cancer Inst. 94, 1247–1249 (2002).
Frye, R. F. et al. Effect of St John's wort on imatinib mesylate pharmacokinetics. Clin. Pharmacol. Ther. 76, 323–329 (2004).
Goey, A. K. et al. The effect of St John's wort on the pharmacokinetics of docetaxel. Clin. Pharmacokinet. 53, 103–110 (2014).
Broxterman, H. J., Lankelma, J. & Hoekman K. Resistance to cytotoxic and anti-angiogenic anticancer agents: similarities and differences. Drug Resist. Updat. 6, 111–127 (2003).
Hamilton, M. et al. Effects of smoking on the pharmacokinetics of erlotinib. Clin. Cancer Res. 12, 2166–2171 (2006).
Hughes, A. N. et al. Overcoming CYP1A1/1A2 mediated induction of metabolism by escalating erlotinib dose in current smokers. J. Clin. Oncol. 27, 1220–1226 (2009).
Van der Bol, J. M. et al. Cigarette smoking and irinotecan treatment: pharmacokinetic interaction and effects on neutropenia. J. Clin. Oncol. 25, 2719–2726 (2007).
Van Erp, N. P. et al. Effect of cigarette smoking on imatinib in patients in the soft tissue and bone sarcoma group of the EORTC. Clin. Cancer Res. 14, 8308–8313 (2008).
De Graan, A. J. et al. Influence of smoking on the pharmacokinetics and toxicity profiles of taxane therapy. Clin. Cancer Res. 18, 4425–4432 (2012).
National Cancer Institute. Cancer.gov[online], (2013).
US National Library of Medicine. Clinicaltrials.gov[online], (2014).
Benowitz, N. L. Cigarette smoking and the personalization of irinotecan therapy. J. Clin. Oncol. 25, 2646–2647 (2007).
Eechoute, K. et al. A long-term prospective population pharmacokinetic study on imatinib plasma concentrations in GIST patients. Clin. Cancer Res. 18, 5780–5787 (2012).
Walko, C. M. & McLeod, H. L. Will we ever be ready for blood level-guided therapy? J. Clin. Oncol. 26, 2078–2079 (2008).
Klümpen, H. J. et al. Moving towards dose individualization of tyrosine kinase inhibitors. Cancer Treat. Rev. 37, 251–260 (2011).
Gamelin, E. et al. Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: results of a multicenter randomized trial of patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 2099–2105 (2008).
Park, J. R. et al. Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high-risk neuroblastoma: a Children's Oncology Group study. J. Clin. Oncol. 29, 4351–4357 (2011).
Lankheet, N. A. G. et al. Individual PK-guided sunitinib dosing: A feasibility study in patients with advanced solid tumors [abstract]. J. Clin. Oncol. 30 (Suppl.), a2596 (2012).
Guchelaar, H. J. et al. Pharmacogenetics in the cancer clinic: from candidate gene studies to next generation sequencing. Clin. Pharmacol. Ther. http://dx.doi.org/10.1038/clpt.2014.13.
Verweij, J. “No Risk, no fun”: challenges for the oncology phase I clinical trial time-performance. The 2008 Michel Clavel Lecture. Eur. J. Cancer 44, 2600–2607 (2008).
Hamberg, P. & Verweij, J. Phase I drug combination trial design: walking the tightrope. J. Clin. Oncol. 27, 4441–4443 (2009).
Hamberg, P., Ratain, M. J., Lesaffre, E. & Verweij, J. Dose-escalation models for combination phase I trials in oncology. Eur. J. Cancer 46, 2870–2878 (2010).
Acknowledgements
This work was supported in part by the American Lebanese Syrian Associated Charities (ALSAC) and National Institutes of Health grant NCI 5R01CA151633-04 (granted to A.S.)
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, contributed substantially to the discussion of content, wrote the manuscript, and edited the manuscript before submission and revised the article after peer review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Mathijssen, R., Sparreboom, A. & Verweij, J. Determining the optimal dose in the development of anticancer agents. Nat Rev Clin Oncol 11, 272–281 (2014). https://doi.org/10.1038/nrclinonc.2014.40
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2014.40
This article is cited by
-
Protein degraders enter the clinic — a new approach to cancer therapy
Nature Reviews Clinical Oncology (2023)
-
One dose to treat them all? – Therapeutisches Drug Monitoring zur Dosisoptimierung in der oralen Antitumortherapie
best practice onkologie (2023)
-
Therapeutic Drug Monitoring of Kinase Inhibitors in Oncology
Clinical Pharmacokinetics (2023)
-
CYP3A4*22 Genotype-Guided Dosing of Kinase Inhibitors in Cancer Patients
Clinical Pharmacokinetics (2023)
-
Bioimaging guided pharmaceutical evaluations of nanomedicines for clinical translations
Journal of Nanobiotechnology (2022)