Key Points
-
A variety of tumour types can develop in the sinonasal cavities; most common are sinonasal squamous-cell carcinoma (SNSCC) and intestinal-type adenocarcinoma (ITAC), which arise in separate sinonasal sublocalizations
-
Patients with sinonasal tumours often present with nonspecific clinical symptoms and with advanced-stage tumours, and therefore, have a poor prognosis
-
Exposure to wood and leather dusts is a strong aetiological factor associated with the development of SNSCC and especially ITAC, possibly through tumorigenic pathways of chronic inflammation
-
SNSCC and ITAC have aneuploid genomes—harbouring multiple genetic aberrations—that are distinct from each other and from histologically similar tumours (head and neck squamous-cell carcinoma and colorectal adenocarcinoma, respectively)
-
Genetic profiling of sinonasal tumours and studies in relevant in vitro cell culture and animal models are laying the foundations for future targeted therapies
-
The clinical management of sinonasal cancer has improved greatly owing to developments in endoscopic surgery and precision radiotherapy
Abstract
The sinonasal cavities represent an anatomical region affected by a variety of tumours with clinical, aetiological, pathological, and genetic features distinct from tumours at the main head and neck cancer localizations. Together, squamous-cell carcinoma and adenocarcinoma account for 80% of all sinonasal tumours, and are aetiologically associated with professional exposure to wood and leather dust particles and other industrial compounds, and therefore, are officially recognized as an occupational disease. Owing to their distinctive characteristics, sinonasal tumours should be considered as separate entities, not to be included in the miscellany of head and neck cancers. Sinonasal tumours are rare, with an annual incidence of approximately 1 case per 100,000 inhabitants worldwide, a fact that has hampered molecular-genetic studies of the tumorigenic pathways and the testing of alternative treatment strategies. Nevertheless, the clinical management of sinonasal cancer has improved owing to advances in imaging techniques, endoscopic surgical approaches, and radiotherapy. Genetic profiling and the development of in vitro cell lines and animal models currently form the basis for future targeted anticancer therapies. We review these advances in our understanding and treatment of sinonasal tumours.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Turner, J. H. & Reh, D. D. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck 34, 877–885 (2012).
Ansa, B. et al. Paranasal sinus squamous cell carcinoma incidence and survival based on Surveillance, Epidemiology, and End Results data, 1973 to 2009. Cancer 119, 2602–2610 (2013).
Mensi, C. et al. Sinonasal cancer and occupational exposure in a population-based registry. Int. J. Otolaryngol. 2013, 672621 (2013).
Kuijpens, J. H. et al. Trends in sinonasal cancer in the Netherlands: more squamous cell cancer, less adenocarcinoma. A population-based study 1973–2009. Eur. J. Cancer 48, 2369–2374 (2012).
Youlden, D. R. et al. International comparisons of the incidence and mortality of sinonasal cancer. Cancer Epidemiol. 37, 770–779 (2013).
Choussy, O. et al. Adenocarcinoma of ethmoid: a GETTEC retrospective multicenter study of 418 cases. Laryngoscope 118, 437–443 (2008).
Sanghvi, S. et al. Epidemiology of sinonasal squamous cell carcinoma: a comprehensive analysis of 4,994 patients. Laryngoscope 124, 76–83 (2014).
Hoppe, B. S. et al. Treatment of nasal cavity and paranasal sinus cancer with modern radiotherapy techniques in the postoperative setting—the MSKCC experience. Int. J. Radiat. Oncol. Biol. Phys. 67, 691–702 (2007).
Cantu, G. et al. Intestinal type adenocarcinoma of the ethmoid sinus in wood and leather workers: a retrospective study of 153 cases. Head Neck 33, 535–542 (2011).
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. IARC Monogr. Eval. Carcinog. Risks Hum. 100, 11–465 (2012).
Hayes, R. B., Gerin, M., Raatgever, J. W. & de Bruyn, A. Wood-related occupations, wood dust exposure, and sinonasal cancer. Am. J. Epidemiol. 124, 569–577 (1986).
Bonzini, M. et al. Prevalence of occupational hazards in patients with different types of epithelial sinonasal cancers. Rhinology 51, 31–36 (2013).
World Health Organization International Agency for Reseach on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans: Wood dust and formaldehyde Vol. 62 (IARC press, 1995).
NIH News Release. New Federal “Report on Carcinogens” Lists Estrogen Therapy, Ultraviolet, Wood Dust [online], (2002).
Blot, W. J., Chow, W. H. & McLaughlin, J. K. Wood dust and nasal cancer risk. A review of the evidence from North America. J. Occup. Environ. Med. 39, 148–156 (1997).
World Health Organization International Agency for Reseach on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Tobacco smoke and involuntary smoking Vol. 83 (IARC Press, 2004).
Syrjänen, K. & Syrjänen, S. Detection of human papillomavirus in sinonasal papillomas: systematic review and meta-analysis. Laryngoscope 123, 181–192 (2013).
Bishop, J. A. et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am. J. Surg. Pathol. 37, 185–192 (2013).
Cantu, G. et al. Anterior craniofacial resection for malignant paranasal tumors: a monoinstitutional experience of 366 cases. Head Neck 34, 78–87 (2012).
Bhayani, M. K. et al. Sinonasal adenocarcinoma: a 16-year experience at a single institution. Head Neck http://dx.doi.org/10.1002/hed.23485.
Haerle, S. K., Gullane, P. J., Witterick, I. J., Zweifel, C. & Gentili, F. Sinonasal carcinomas: epidemiology, pathology, and management. Neurosurg. Clin. N. Am. 24, 39–49 (2013).
Lamarre, E. D. et al. Role of positron emission tomography in management of sinonasal neoplasms—a single institution's experience. Am. J. Otolaryngol. 33, 289–295 (2012).
Sobin, L. H., Gospodarowicz, M. K. & Wittekind, C. H. (eds) International Union Against Cancer (UICC) TNM Classification of Malignant Tumors 7th edn (Wiley-Blackwell, 2009).
Barnes, L., Eveson, J. W., Reichart, P. & Sidransky, D. (eds) World Health Organization Classification of Tumours Vol. 9: Pathology and Genetics of Head and Neck Tumours (IARC Press, 2005).
Nazar, G., Rodrigo, J. P., Llorente, J. L., Baragaño, L. & Suárez, C. Prognostic factors of maxillary sinus malignancies. Am. J. Rhinol. 18, 233–238 (2004).
Kang, J. H. et al. Treatment outcomes between concurrent chemoradiotherapy and combination of surgery, radiotherapy, and/or chemotherapy in stage III and IV maxillary sinus cancer: multi-institutional retrospective analysis. J. Oral Maxillofac. Surg. 70, 1717–1723 (2012).
Ben-Neriah, Y. & Karin, M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat. Immunol. 12, 715–723 (2011).
Määttä, J. et al. Characterization of oak and birch dust-induced expression of cytokines and chemokines in mouse macrophage RAW 264.7 cells. Toxicology 215, 25–36 (2005).
Holmila, R. et al. COX-2 and p53 in human sinonasal cancer: COX-2 expression is associated with adenocarcinoma histology and wood-dust exposure. Int. J. Cancer 122, 2154–2159 (2008).
Pylkkänen, L., Stockmann-Juvala, H., Alenius, H., Husgafvel-Pursiainen, K. & Savolainen, K. Wood dusts induce the production of reactive oxygen species and caspase-3 activity in human bronchial epithelial cells. Toxicology 262, 265–270 (2009).
Hold, G. L. & El-Omar, E. M. Genetic aspects of inflammation and cancer. Biochem. J. 410, 225–235 (2008).
Pérez-Escuredo, J. et al. Wood dust-related mutational profile of TP53 in intestinal-type sinonasal adenocarcinoma. Hum. Pathol. 43, 1894–1901 (2012).
Perrone, F. et al. TP53, p14ARF, p16INK4a and H-RAS gene molecular analysis in intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Int. J. Cancer 105, 196–203 (2003).
Holmila, R. et al. Mutations in TP53 tumor suppressor gene in wood dust-related sinonasal cancer. Int. J. Cancer 127, 578–588 (2010).
Bornholdt, J. et al. K-RAS mutations in sinonasal cancers in relation to wood dust exposure. BMC Cancer 8, 53 (2008).
Mackinnon, A. C. Jr et al. Cribriform adenocarcinoma of the lung: clinicopathologic, immunohistochemical, and molecular analysis of 15 cases of a distinctive morphologic subtype of lung adenocarcinoma. Mod. Pathol. http://dx.doi.org/10.1038/modpathol.2013.227.
Shen, B. et al. Human defensin 5 expression in intestinal metaplasia of the upper gastrointestinal tract. J. Clin. Pathol. 58, 687–694 (2005).
Choi, H. R. et al. Sinonasal adenocarcinoma: evidence for histogenetic divergence of the enteric and nonenteric phenotypes. Hum. Pathol. 34, 1101–1107 (2003).
Valente, G. et al. Evidence of p53 immunohistochemical overexpression in ethmoidal mucosa of woodworkers. Cancer Detect. Prev. 28, 99–106 (2004).
Vivanco, B. et al. Benign lesions in mucosa adjacent to intestinal-type sinonasal adenocracinoma. Patholog. Res. Int. 2011, 230147 (2011).
Kennedy, M. T., Jordan, R. C., Berean, K. W. & Perez-Ordoñez, B. Expression pattern of CK7, CK20, CDX-2, and villin in intestinal-type sinonasal adenocarcinoma. J. Clin. Pathol. 57, 932–937 (2004).
Cabanillas, R. & Llorente, J. L. The Stem Cell Network model: clinical implications in cancer. Eur. Arch. Otorhinolaryngol. 266, 161–170 (2009).
Chinn, S. B. et al. Cancer stem cells mediate tumorigenesis and metastasis in head and neck squamous cell carcinoma. Head Neck http://dx.doi.org/10.1002/hed.23600.
Leung, C. T., Coulombe, P. A. & Reed, R. R. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat. Neurosci. 10, 720–726 (2007).
Hauser, S. et al. Isolation of novel multipotent neural crest-derived stem cells from adult human inferior turbinate. Stem Cells Dev. 21, 742–756 (2012).
Jankowski, R. Revisiting human nose anatomy: phylogenic and ontogenic perspectives. Laryngoscope 121, 2461–2467 (2011).
Georgel, T. et al. CT assessment of woodworkers' nasal adenocarcinomas confirms the origin in the olfactory cleft. Am. J. Neuroradiol. 30, 1440–1444 (2009).
Costa, A. F. et al. Analysis of MYB oncogene in transformed adenoid cystic carcinomas reveals distinct pathways of tumor progression. Lab. Invest. 94, 692–702 (2014).
Franchi, A. et al. Primary combined neuroendocrine and squamous cell carcinoma of the maxillary sinus: report of a case with immunohistochemical and molecular characterization. Head Neck Pathol. http://dx.doi.org/10.1007/s12105-013-0513-5.
Kang, S. Y., McHugh, J. B., Sullivan, S. E., Marentette, L. J. & McKean, E. L. Sinonasal undifferentiated carcinoma and esthesioneuroblastoma recurring as nonintestinal adenocarcinoma. Laryngoscope 123, 1121–1124 (2013).
Valent, P. et al. Cancer stem cell definitions and terminology: the devil is in the details. Nat. Rev. Cancer. 12, 767–775 (2012).
Barcellos-Hoff, M. H., Lyden, D. & Wang, T. C. The evolution of the cancer niche during multistage carcinogenesis. Nat. Rev. Cancer 13, 511–518 (2013).
Visvader, J. E. & Lindeman, G. J. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer 8, 755–768 (2008).
Zhang, Z., Filho, M. S. & Nör, J. E. The biology of head and neck cancer stem cells. Oral Oncol. 48, 1–9 (2012).
Sayed, S. I. et al. Implications of understanding cancer stem cell (CSC) biology in head and neck squamous cell cancer. Oral Oncol. 47, 237–243 (2011).
Reers, S., Pfannerstill, A. C., Maushagen, R., Pries, R. & Wollenberg, B. Stem cell profiling in head and neck cancer reveals an Oct-4 expressing subpopulation with properties of chemoresistance. Oral Oncol. 50, 155–162 (2014).
Bossi, P. et al. TP53 status as guide for the management of ethmoid sinus intestinal-type adenocarcinoma. Oral Oncol. 49, 413–419 (2013).
Yom, S. S. et al. Genetic analysis of sinonasal adenocarcinoma phenotypes: distinct alterations of histogenetic significance. Mod. Pathol. 18, 315–319 (2005).
Takahashi, Y. et al. Comprehensive assessment of prognostic markers for sinonasal squamous cell carcinoma. Head Neck http://dx.doi.org/10.1002/hed.23423.
Franchi, A. et al. Immunohistochemical investigation of tumorigenic pathways in sinonasal intestinal-type adenocarcinoma. A tissue microarray analysis of 62 cases. Histopathology. 59, 98–105 (2011).
Díaz-Molina, J. P. et al. Wnt-pathway activation in intestinal-type sinonasal adenocarcinoma. Rhinology 49, 593–599 (2011).
Perez-Ordonez, B., Huynh, N. N., Berean, K. W. & Jordan, R. C. K. Expression of mismatch repair proteins, β-catenin, and E-cadherin in intestinal-type sinonasal adenocarcinoma. J. Clin. Pathol. 57, 1080–1083 (2005).
Claessen, M. M. et al. Wnt-pathway activation in IBD-associated colorectal carcinogenesis: potential biomarkers for colonic surveillance. Cell. Oncol. 32, 303–310 (2010).
Shenoy, A. K. et al. Transition from colitis to cancer: high Wnt activity sustains the tumor-initiating potential of colon cancer stem cell precursors. Cancer Res. 72, 5091–5100 (2012).
Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012).
García-Inclán, C. et al. EGFR status and KRAS/BRAF mutations in intestinal-type sinonasal adenocarcinomas. Cell. Oncol. (Dordr.) 35, 443–450 (2012).
Franchi, A. et al. Epidermal growth factor receptor expression and gene copy number in sinonasal intestinal type adenocarcinoma. Oral Oncol. 45, 835–838 (2009).
López, F. et al. Gene amplification and protein overexpression of EGFR and ERBB2 in sinonasal squamous cell carcinoma. Cancer 118, 1818–1826 (2012).
Temam, S. et al. Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. J. Clin. Oncol. 25, 2164–2170 (2007).
Ljuslinder, I. et al. Increased epidermal growth factor receptor expression at the invasive margin is a negative prognostic factor in colorectal cancer. Int. J. Cancer 128, 2031–2037 (2011).
López, F. et al. KRAS and BRAF mutations in sinonasal cancer. Oral Oncol. 48, 692–697 (2012).
Frattini, M. et al. Phenotype-genotype correlation: challenge of intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Head Neck 28, 909–915 (2006).
Schröck, A. et al. Fibroblast-growth-factor-receptor-1 as a potential therapeutic target in sinonasal cancer? Head Neck http://dx.doi.org/10.1002/hed.23443.
Martínez, J. G. et al. Microsatellite instability analysis of sinonasal carcinomas. Otolaryngol. Head Neck Surg. 140, 55–60 (2009).
Schröck, A. et al. Sex determining region Y-Box 2 (SOX2) amplification is an independent indicator of disease recurrence in sinonasal cancer. PLoS ONE 8, e59201 (2013).
Hermsen, M. A. et al. Genome-wide analysis of genetic changes in intestinal-type sinonasal adenocarcinoma. Head Neck 31, 290–297 (2009).
Korinth, D. et al. Chromosomal imbalances in wood dust-related adenocarcinomas of the inner nose and their associations with pathological parameters. J. Pathol. 207, 207–215 (2005).
López, F. et al. Genomic profiling of sinonasal squamous cell carcinoma. Head Neck 33, 145–153 (2011).
Ariza, M. et al. Comparative genomic hybridization of primary sinonasal adenocarcinomas. Cancer 100, 335–341 (2004).
Persson, M. et al. Clinically significant copy number alterations and complex rearrangements of MYB and NFIB in head and neck adenoid cystic carcinoma. Genes Chromosomes Cancer 51, 805–817 (2012).
Leemans, C. R., Braakhuis, B. J. & Brakenhoff, R. H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 11, 9–22 (2011).
Tripodi, D. et al. Gene expression profiling in sinonasal adenocarcinoma. BMC Med. Genomics 2, 65 (2009).
Stransky, N. et al. The mutational landscape of head and neck squamous cell carcinoma. Science 333, 1157–1160 (2011).
Pérez-Escuredo, J. et al. Establishment and genetic characterization of an immortal tumor cell line derived from intestinal-type sinonasal adenocarcinoma. Cell. Oncol. (Dordr.) 34, 23–31 (2011).
García-Inclán, C. et al. Establishment and genetic characterization of six unique tumor cell lines as preclinical models for sinonasal squamous cell carcinoma. Sci. Rep. 4, 4925 (2014).
Takahashi, Y. et al. Establishment and characterization of novel cell lines from sinonasal undifferentiated carcinoma. Clin. Cancer Res. 18, 6178–6187 (2012).
Lund, V. J. et al. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol. Suppl. 22, 1–143 (2010).
Su, S. Y. et al. Endoscopic resection of sinonasal cancers. Curr. Oncol. Rep. 16, 369 (2014).
Snyderman, C. H. et al. Endoscopic skull base surgery: principles of endonasal oncological surgery. J. Surg. Oncol. 97, 658–664 (2008).
Llorente, J. L. et al. Outcomes following microvascular free tissue transfer in reconstructing skull base defects. J. Neurol. Surg. B Skull Base. 74, 324–330 (2013).
Ketcham, A. S., Wilkins, R. H., Vanburen, J. M. & Smith, R. R. A combined intracranial facial approach to the paranasal sinuses. Am. J. Surg. 106, 698–703 (1963).
Castelnuovo, P., Dallan, I., Battaglia, P. & Bignami, M. Endoscopic endonasal skull base surgery: past, present and future. Eur. Arch. Otorhinolaryngol. 267, 649–663 (2010).
Ambrosch, P. The role of laser microsurgery in the treatment of laryngeal cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 15, 82–88 (2007).
Kassam, A., Snyderman, C. H., Carrau, R. L., Gardner, P. & Mintz, A. Endoneurosurgical hemostasis techniques: lessons learned from 400 cases. Neurosurg. Focus 19, E7 (2005).
Hadad, G. et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 116, 1882–1886 (2006).
Nicolai, P., Villaret, A. B., Bottazzoli, M., Rossi, E. & Valsecchi, M. G. Ethmoid adenocarcinoma—from craniofacial to endoscopic resections: a single-institution experience over 25 years. Otolaryngol. Head Neck Surg. 145, 330–337 (2011).
Kasemsiri, P. et al. Endoscopic endonasal technique: treatment of paranasal and anterior skull base malignancies. Braz. J. Otorhinolaryngol. 79, 760–779 (2013).
Higgins, T. S., Thorp, B., Rawlings, B. A. & Han, J. K. Outcome results of endoscopic vs craniofacial resection of sinonasal malignancies: a systematic review and pooled-data analysis. Int. Forum Allergy Rhinol. 1, 255–261 (2011).
Castelnuovo, P. et al. Quality of life following endoscopic endonasal resection of anterior skull base cancers. J. Neurosurg. 119, 1401–1409 (2013).
Eichhorn, K. W. & Bootz, F. Clinical requirements and possible applications of robot assisted endoscopy in skull base and sinus surgery. Acta Neurochir. Suppl. 109, 237–240 (2011).
Spratt, D., Cabanillas, R. & Lee, N. Y. The Paranasal Sinuses in Target Volume Delineation and Field Setup in A Practical Guide for Conformal and Intensity-Modulated Radiation Therapy (eds Lee, N. J. & Lu, J. J.) 45–49 (Springer, 2013).
Duprez, F. et al. IMRT for sinonasal tumors minimizes severe late ocular toxicity and preserves disease control and survival. Int. J. Radiat. Oncol. Biol. Phys. 83, 252–259 (2012).
Spiotto, M. T. & Weichselbaum, R. R. Comparison of 3D conformal radiotherapy and intensity modulated radiotherapy with or without simultaneous integrated boost during concurrent chemoradiation for locally advanced head and neck cancers. PLoS ONE 9, e94456 (2014).
Marta, G. N. et al. Intensity-modulated radiation therapy for head and neck cancer: systematic review and meta-analysis. Radiother. Oncol. 110, 9–15 (2014).
Alonso-Basanta, M., Lustig, R. A. & Kennedy, D. W. Proton beam therapy in skull base pathology. Otolaryngol. Clin. North Am. 44, 1173–1183 (2011).
Chen, A. M. et al. Utility of daily image guidance with intensity-modulated radiotherapy for tumors of the base of skull. Head Neck 34, 763–770 (2012).
Hanna, E. Y. et al. Induction chemotherapy for advanced squamous cell carcinoma of the paranasal sinuses. Arch. Otolaryngol. Head Neck Surg. 137, 78–81 (2011).
Homma, A. et al. Superselective high dose cisplatin infusion with concomitant radiotherapy in patients with advanced cancer of the nasal cavity and paranasal sinuses: a single institution experience. Cancer 115, 4705–4714 (2009).
Papadimitrakopoulou, V. A. et al. Intraarterial cisplatin with intravenous paclitaxel and ifosfamide as an organ-preservation approach in patients with paranasal sinus carcinoma. Cancer 98, 2214–2223 (2003).
Hoppe, B. S. et al. Unresectable carcinoma of the paranasal sinuses: outcomes and toxicities. Int. J. Radiat. Oncol. Biol. Phys. 72, 763–769 (2008).
Rosen, A. et al. Locoregionally advanced paranasal sinus carcinoma. Favorable survival with multimodality therapy. Arch. Otolaryngol. Head Neck Surg. 119, 743–746 (1993).
Lee, M. M. et al. Multimodality therapy in advanced paranasal sinus carcinoma: superior long-term results. Cancer J. Sci. Am. 5, 219–223 (1999).
Kies, M. S. et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J. Clin. Oncol. 28, 8–14 (2010).
Knegt, P. P., Ah-See, K. W., vd Velden, L. A. & Kerrebijn, J. Adenocarcinoma of the ethmoidal sinus complex: surgical debulking and topical fluorouracil may be the optimal treatment. Arch. Otolaryngol. Head Neck Surg. 127, 141–146 (2001).
Choi, I. J., Kim, D. W., Kim, D. Y., Lee, C. H. & Rhee, C. S. Predictive markers for neoadjuvant chemotherapy in advanced squamous cell carcinoma of maxillary sinus: preliminary report. Acta Otolaryngol. 133, 291–296 (2013).
Bandoh, N. et al. VEGF and bFGF expression and microvessel density of maxillary sinus squamous cell carcinoma in relation to p53 status, spontaneous apoptosis and prognosis. Cancer Lett. 208, 215–225 (2004).
Acknowledgements
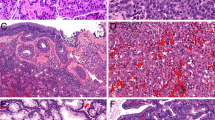
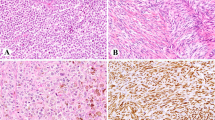
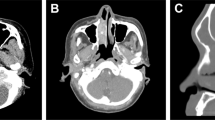
The authors thank R. Cabanillas for his assistance with radiotherapy issues and images, B. Vivanco for preparing the pathological images shown herein, and J. Pérez-Escuredo, C. García-Inclán and C. Álvarez-Marcos for their active collaboration.
Author information
Authors and Affiliations
Contributions
J.L.L., F.L. and M.A.H. contributed to all stages of the preparation of the manuscript. C.S. made substantial contributions to discussion of content and review/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Video 1
Endoscopic surgery of an ITAC originating in the ethmoid sinus. This video provides an overview of a craniofacial endoscopic approach to resect a bilateral ethmoid ITAC. After tumour debulking and drilling the surrounding bone to reach the margins, the tumour implantation site is identified in the olfactory cleft. Osteotomies and monobloc resection, including the dura mater, are performed around the cribiform plates from the frontal sinuses to the planum sphenoidale. The defect created in the dura mater is repaired by grafting two layers of fascia lata tissue to separate the brain. Ultimately, a clean cavity is left, demarcated by the periorbita of both eyes and a roof formed of the grafted fascia lata tissue. Abbreviations: ITAC, intestinal-type adenocarcinoma. (AVI 16204 kb)
Rights and permissions
About this article
Cite this article
Llorente, J., López, F., Suárez, C. et al. Sinonasal carcinoma: clinical, pathological, genetic and therapeutic advances. Nat Rev Clin Oncol 11, 460–472 (2014). https://doi.org/10.1038/nrclinonc.2014.97
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2014.97
This article is cited by
-
Choice of surgery in intestinal-type adenocarcinoma of the sinonasal tract: a long-term comparative study
European Archives of Oto-Rhino-Laryngology (2024)
-
Clinicopathological study and management of masses in the sinonasal cavity and nasopharynx: a case series of 42 cases
The Egyptian Journal of Otolaryngology (2023)
-
SWI/SNF-Deficient Sinonasal Carcinomas: Multidisciplinary Research Perspectives
Current Otorhinolaryngology Reports (2023)
-
Experimental Models of Sinonasal Tumors for Preclinical Testing of Candidate Targeted Therapies
Current Otorhinolaryngology Reports (2023)
-
Diffuse spinal cord metastasis after resection of SMARCB1 sinonasal carcinoma manifesting with a right foot drop—a case report
Spinal Cord Series and Cases (2022)