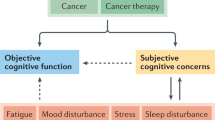
Key Points
-
Preclinical and clinical data indicate that endocrine treatment for breast cancer might have an adverse effect on cognition
-
Many studies exploring the influence of endocrine therapy on cognition are underpowered and have flawed designs, precluding any definite conclusions on the existence and clinical impact of such effects
-
Studies with a pretreatment neuropsychological assessment are essential to determine the potential cognitive effects of endocrine treatment and to identify patients who might be at risk of treatment-associated cognitive decline
-
Current guidelines permit the choice between different endocrine regimens in the treatment of breast cancer; thus, potential treatment-selective cognitive effects might influence treatment decision-making on an individualized basis
Abstract
The number of breast cancer survivors is gradually increasing and a subset of these patients experience long-term adverse effects of adjuvant systemic therapy, including cognitive decline. Surprisingly, relatively little is known about the long-term adverse effects of endocrine treatment on cognition. As 75% of all patients with breast cancer are eligible to receive hormonal treatment, understanding the potential neurocognitive adverse effects of such therapy is of utmost importance. Concerns about adverse cognitive effects of adjuvant endocrine therapy are timely, as recently updated guidelines recommend increasing the length of such therapy from 5 years to 10 years for a subset of patients. The decline of cognitive functions can have a detrimental impact on quality of life and might interfere with independent living. This Review discusses the tissue-selective side effects of endocrine therapies and specifically their impact on cognitive function, on the basis of clinical data; the neurobiological effects of endocrine therapies as observed in preclinical models are also discussed. We highlight the critical issues that need to be addressed in future preclinical and clinical studies in order to best assess the cognitive effects of endocrine treatment in patients with breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kamangar, F., Dores, G. M. & Anderson, W. F. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 24, 2137–2150 (2006).
Coombes, R. C. et al. Survival and safety of exemestane versus tamoxifen after 2–3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet 369, 559–570 (2007).
Jakesz, R. et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 366, 455–462 (2005).
Baum, M. et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359, 2131–2139 (2002).
van de Velde, C. J. et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet 377, 321–331 (2011).
Breast International Group 1–98 Collaborative, Group et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N. Engl. J. Med. 353, 2747–2757 (2005).
Harbeck, N., Sotlar, K., Wuerstlein, R. & Doisneau-Sixou, S. Molecular and protein markers for clinical decision making in breast cancer: today and tomorrow. Cancer Treat. Rev. 40, 434–444 (2014).
Goldhirsch, A. et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 24, 2206–2223 (2013).
Smith, G. L. The long and short of tamoxifen therapy: a review of the ATLAS trial. J. Adv. Pract. Oncol. 5, 57–60 (2014).
Davies, C. et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381, 805–816 (2013).
Jakesz, R. et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J. Natl Cancer Inst. 99, 1845–1853 (2007).
Goss, P. E. et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N. Engl. J. Med. 349, 1793–1802 (2003).
Dubsky, P. et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2− breast cancer patients. Br. J. Cancer 109, 2959–2964 (2013).
Filipits, M. et al. The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clin. Cancer Res. 20, 1298–1305 (2014).
Sestak, I. et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J. Clin. Oncol. 33, 916–922 (2015).
Wefel, J. S., Kesler, S. R., Noll, K. R. & Schagen, S. B. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J. Clin. 65, 123–138 (2015).
Reid-Arndt, S. A., Yee, A., Perry, M. C. & Hsieh, C. Cognitive and psychological factors associated with early posttreatment functional outcomes in breast cancer survivors. J. Psychosoc. Oncol. 27, 415–434 (2009).
de Ruiter, M. B. et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum. Brain Mapp. 33, 2971–2983 (2012).
Mandelblatt, J. S., Jacobsen, P. B. & Ahles, T. Cognitive effects of cancer systemic therapy: implications for the care of older patients and survivors. J. Clin. Oncol. 32, 2617–2626 (2014).
O'Farrell, E., MacKenzie, J. & Collins, B. Clearing the air: a review of our current understanding of “chemo fog”. Curr. Oncol. Rep. 15, 260–269 (2013).
Vearncombe, K. J. et al. Cognitive effects of chemotherapy-induced menopause in breast cancer. Clin. Neuropsychol. 25, 1295–1313 (2011).
Schagen, S. B., Muller, M. J., Boogerd, W., Mellenbergh, G. J. & van Dam, F. S. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. J. Natl Cancer Inst. 98, 1742–1745 (2006).
Conroy, S. K., McDonald, B. C., Ahles, T. A., West, J. D. & Saykin, A. J. Chemotherapy-induced amenorrhea: a prospective study of brain activation changes and neurocognitive correlates. Brain Imaging Behav. 7, 491–500 (2013).
Jenkins, V. et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br. J. Cancer 94, 828–834 (2006).
Castellon, S. A. et al. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J. Clin. Exp. Neuropsychol. 26, 955–969 (2004).
Collins, B., Mackenzie, J., Stewart, A., Bielajew, C. & Verma, S. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psychooncology 18, 134–143 (2009).
Arevalo, M. A., Azcoitia, I. & Garcia-Segura, L. M. The neuroprotective actions of oestradiol and oestrogen receptors. Nat. Rev. Neurosci. 16, 17–29 (2014).
Esmaeili, B., Basseda, Z., Gholizadeh, S., Javadi Paydar, M. & Dehpour, A. R. Tamoxifen disrupts consolidation and retrieval of morphine-associated contextual memory in male mice: interaction with estradiol. Psychopharmacology (Berl.) 204, 191–201 (2009).
Phillips, K. A., Ribi, K. & Fisher, R. Do aromatase inhibitors have adverse effects on cognitive function? Breast Cancer Res. 13, 203 (2011).
McEwen, B. S., Akama, K. T., Spencer-Segal, J. L., Milner, T. A. & Waters, E. M. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav. Neurosci. 126, 4–16 (2012).
Azcoitia, I., Yague, J. G. & Garcia-Segura, L. M. Estradiol synthesis within the human brain. Neuroscience 191, 139–147 (2011).
Velazquez-Zamora, D. A. et al. Plastic changes in dendritic spines of hippocampal CA1 pyramidal neurons from ovariectomized rats after estradiol treatment. Brain Res. 1470, 1–10 (2012).
Fester, L. et al. Estrogen-regulated synaptogenesis in the hippocampus: sexual dimorphism in vivo but not in vitro. J. Steroid Biochem. Mol. Biol. 131, 24–29 (2012).
Brueggemeier, R. W. Aromatase inhibitors: new endocrine treatment of breast cancer. Semin. Reprod. Med. 22, 31–43 (2004).
Fester, L., Prange-Kiel, J., Jarry, H. & Rune, G. M. Estrogen synthesis in the hippocampus. Cell Tissue Res. 345, 285–294 (2011).
Ito, K. et al. A case of brain metastases from breast cancer that responded to anastrozole monotherapy. Breast J. 15, 435–437 (2009).
Kil, K. E. et al. Synthesis and PET studies of [(11)C-cyano]letrozole (Femara), an aromatase inhibitor drug. Nucl. Med. Biol. 36, 215–223 (2009).
Lien, E. A., Solheim, E. & Ueland, P. M. Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer Res. 51, 4837–4844 (1991).
Gonzalez, M. et al. Distribution patterns of estrogen receptor alpha and beta in the human cortex and hippocampus during development and adulthood. J. Comp. Neurol. 503, 790–802 (2007).
Zwart, W. et al. The hinge region of the human estrogen receptor determines functional synergy between AF1 and AF2 in the quantitative response to estradiol and tamoxifen. J. Cell Sci. 123, 1253–1261 (2010).
McInerney, E. M., Weis, K. E., Sun, J., Mosselman, S. & Katzenellenbogen, B. S. Transcription activation by the human estrogen receptor subtype beta (ER beta) studied with ER beta and ER alpha receptor chimeras. Endocrinology 139, 4513–4522 (1998).
Hall, J. M. & McDonnell, D. P. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140, 5566–5578 (1999).
Roger, P. et al. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 61, 2537–2541 (2001).
Osterlund, M. K., Gustafsson, J. A., Keller, E. & Hurd, Y. L. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERalpha mRNA. J. Clin. Endocrinol. Metab. 85, 3840–3846 (2000).
Dubal, D. B., Shughrue, P. J., Wilson, M. E., Merchenthaler, I. & Wise, P. M. Estradiol modulates bcl2 in cerebral ischemia: a potential role for estrogen receptors. J. Neurosci. 19, 6385–6393 (1999).
Mitterling, K. L. et al. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J. Comp. Neurol. 518, 2729–2743 (2010).
Gibbs, R. B. & Johnson, D. A. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology 149, 3176–3183 (2008).
Cao, F. et al. Ovariectomy-mediated impairment of spatial working memory, but not reference memory, is attenuated by the knockout of the dopamine D(3) receptor in female mice. Behav. Brain Res. 247, 27–33 (2013).
Li, C. et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc. Natl Acad. Sci. USA 101, 2185–2190 (2004).
Fernandez, S. M. et al. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J. Neurosci. 28, 8660–8667 (2008).
Jasnow, A. M., Schulkin, J. & Pfaff, D. W. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm. Behav. 49, 197–205 (2006).
Chen, D., Wu, C. F., Shi, B. & Xu, Y. M. Tamoxifen and toremifene cause impairment of learning and memory function in mice. Pharmacol. Biochem. Behav. 71, 269–276 (2002).
Mook, D., Felger, J., Graves, F., Wallen, K. & Wilson, M. E. Tamoxifen fails to affect central serotonergic tone but increases indices of anxiety in female rhesus macaques. Psychoneuroendocrinology 30, 273–283 (2005).
Aydin, M. et al. Effects of letrozole on hippocampal and cortical catecholaminergic neurotransmitter levels, neural cell adhesion molecule expression and spatial learning and memory in female rats. Neuroscience 151, 186–194 (2008).
Zhou, L. et al. Aromatase inhibitors induce spine synapse loss in the hippocampus of ovariectomized mice. Endocrinology 151, 1153–1160 (2010).
Grassi, S. et al. Neural 17beta-estradiol facilitates long-term potentiation in the hippocampal CA1 region. Neuroscience 192, 67–73 (2011).
Velazquez-Zamora, D. A., Garcia-Segura, L. M. & Gonzalez-Burgos, I. Effects of selective estrogen receptor modulators on allocentric working memory performance and on dendritic spines in medial prefrontal cortex pyramidal neurons of ovariectomized rats. Horm. Behav. 61, 512–517 (2012).
Sharma, K. & Mehra, R. D. Long-term administration of estrogen or tamoxifen to ovariectomized rats affords neuroprotection to hippocampal neurons by modulating the expression of Bcl2 and Bax. Brain Res. 1204, 1–15 (2008).
McMillan, P. J., LeMaster, A. M. & Dorsa, D. M. Tamoxifen enhances choline acetyltransferase mRNA expression in rat basal forebrain cholinergic neurons. Brain Res. Mol. Brain Res. 103, 140–145 (2002).
Wu, X. et al. Raloxifene and estradiol benzoate both fully restore hippocampal choline acetyltransferase activity in ovariectomized rats. Brain Res. 847, 98–104 (1999).
Silva, I., Mello, L. E., Freymuller, E., Haidar, M. A. & Baracat, E. C. Estrogen, progestogen and tamoxifen increase synaptic density of the hippocampus of ovariectomized rats. Neurosci. Lett. 291, 183–186 (2000).
Vierk, R. et al. Aromatase inhibition abolishes LTP generation in female but not in male mice. J. Neurosci. 32, 8116–8126 (2012).
Couse, J. F. & Korach, K. S. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr. Rev. 20, 358–417 (1999).
Wang, L., Andersson, S., Warner, M. & Gustafsson, J. A. Morphological abnormalities in the brains of estrogen receptor beta knockout mice. Proc. Natl Acad. Sci. USA 98, 2792–2796 (2001).
Harris, H. A. Estrogen receptor-beta: recent lessons from in vivo studies. Mol. Endocrinol. 21, 1–13 (2007).
Gundlah, C., Lu, N. Z., Mirkes, S. J. & Bethea, C. L. Estrogen receptor beta (ERbeta) mRNA and protein in serotonin neurons of macaques. Brain Res. Mol. Brain Res. 91, 14–22 (2001).
Amin, Z., Canli, T. & Epperson, C. N. Effect of estrogen-serotonin interactions on mood and cognition. Behav. Cogn. Neurosci. Rev. 4, 43–58 (2005).
Sumner, B. E. et al. Effects of tamoxifen on serotonin transporter and 5hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res. Mol. Brain Res. 73, 119–128 (1999).
Mize, A. L., Shapiro, R. A. & Dorsa, D. M. Estrogen receptor-mediated neuroprotection from oxidative stress requires activation of the mitogen-activated protein kinase pathway. Endocrinology 144, 306–312 (2003).
Luine, V. N. Estradiol and cognitive function: past, present and future. Horm. Behav. 66, 602–618 (2014).
Toffoletto, S., Lanzenberger, R., Gingnell, M., Sundstrom-Poromaa, I. & Comasco, E. Emotional and cognitive functional imaging of estrogen and progesterone effects in the female human brain: A systematic review. Psychoneuroendocrinology 50, 28–52 (2014).
Sherwin, B. B. Estrogen and cognitive functioning in women: lessons we have learned. Behav. Neurosci. 126, 123–127 (2012).
Sherwin, B. B. & Henry, J. F. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front. Neuroendocrinol. 29, 88–113 (2008).
Sherwin, B. B. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? J. Neuroendocrinol. 19, 77–81 (2007).
Luetters, C. et al. Menopause transition stage and endogenous estradiol and follicle-stimulating hormone levels are not related to cognitive performance: cross-sectional results from the study of women's health across the nation (SWAN). J. Womens Health (Larchmt) 16, 331–344 (2007).
Schmidt, P. J. et al. Cognitive performance in healthy women during induced hypogonadism and ovarian steroid addback. Arch. Womens Ment. Health 16, 47–58 (2013).
Maki, P. M., Rich, J. B. & Rosenbaum, R. S. Implicit memory varies across the menstrual cycle: estrogen effects in young women. Neuropsychologia 40, 518–529 (2002).
Henderson, V. W. & Sherwin, B. B. Surgical versus natural menopause: cognitive issues. Menopause 14, 572–579 (2007).
Drake, E. B. et al. Associations between circulating sex steroid hormones and cognition in normal elderly women. Neurology 54, 599–603 (2000).
Rocca, W. A. et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 69, 1074–1083 (2007).
Phung, T. K. et al. Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dement. Geriatr. Cogn. Disord. 30, 43–50 (2010).
Bove, R. et al. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology 82, 222–229 (2014).
Rocca, W. A., Grossardt, B. R. & Shuster, L. T. Oophorectomy, estrogen, and dementia: a 2014 update. Mol. Cell. Endocrinol. 389, 7–12 (2014).
Ryan, J. et al. Impact of a premature menopause on cognitive function in later life. BJOG 121, 1729–1739 (2014).
Shumaker, S. A. et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 289, 2651–2662 (2003).
Coker, L. H. et al. Change in brain and lesion volumes after CEE therapies: the WHIMS-MRI studies. Neurology 82, 427–434 (2014).
Paganini-Hill, A. & Clark, L. J. Preliminary assessment of cognitive function in breast cancer patients treated with tamoxifen. Breast Cancer Res. Treat. 64, 165–176 (2000).
Maki, P. Is timing everything? New insights into why the effect of estrogen therapy on memory might be age dependent. Endocrinology 154, 2570–2572 (2013).
Eberling, J. L., Wu, C., Tong-Turnbeaugh, R. & Jagust, W. J. Estrogen- and tamoxifen-associated effects on brain structure and function. Neuroimage 21, 364–371 (2004).
Hanssen, K. T., Beiske, A. G., Landro, N. I. & Hessen, E. Predictors of executive complaints and executive deficits in multiple sclerosis. Acta Neurol. Scand. 129, 234–242 (2014).
Lannoo, E. et al. Subjective complaints versus neuropsychological test performance after moderate to severe head injury. Acta Neurochir. (Wien) 140, 245–253 (1998).
Edmonds, E. C., Delano-Wood, L., Galasko, D. R., Salmon, D. P. & Bondi, M. W. Subjective cognitive complaints contribute to misdiagnosis of mild cognitive impairment. J. Int. Neuropsychol. Soc. 20, 836–847 (2014).
Gorman, A. A., Foley, J. M., Ettenhofer, M. L., Hinkin, C. H. & van Gorp, W. G. Functional consequences of HIV-associated neuropsychological impairment. Neuropsychol. Rev. 19, 186–203 (2009).
Hutchinson, A. D., Hosking, J. R., Kichenadasse, G., Mattiske, J. K. & Wilson, C. Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat. Rev. 38, 926–934 (2012).
Cuzick, J. et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet 383, 1041–1048 (2014).
Jenkins, V., Shilling, V., Fallowfield, L., Howell, A. & Hutton, S. Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A pilot study. Psychooncology 13, 61–66 (2004).
Schilder, C. M. et al. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J. Clin. Oncol. 28, 1294–1300 (2010).
Phillips, K. A. et al. Cognitive function in postmenopausal women receiving adjuvant letrozole or tamoxifen for breast cancer in the BIG 1–98 randomized trial. Breast 19, 388–395 (2010).
Phillips, K. A. et al. Cognitive function in postmenopausal breast cancer patients one year after completing adjuvant endocrine therapy with letrozole and/or tamoxifen in the BIG 1–98 trial. Breast Cancer Res. Treat. 126, 221–226 (2011).
Jenkins, V. A. et al. Effects of anastrozole on cognitive performance in postmenopausal women: a randomised, double-blind chemoprevention trial (IBIS II). Lancet Oncol. 9, 953–961 (2008).
Wefel, J. S. & Schagen, S. B. Chemotherapy-related cognitive dysfunction. Curr. Neurol. Neurosci. Rep. 12, 267–275 (2012).
Ahles, T. A., Root, J. C. & Ryan, E. L. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J. Clin. Oncol. 30, 3675–3686 (2012).
Collins, B., Mackenzie, J., Stewart, A., Bielajew, C. & Verma, S. Cognitive effects of hormonal therapy in early stage breast cancer patients: a prospective study. Psychooncology 18, 811–821 (2009).
Lejbak, L., Vrbancic, M. & Crossley, M. Endocrine therapy is associated with low performance on some estrogen-sensitive cognitive tasks in postmenopausal women with breast cancer. J. Clin. Exp. Neuropsychol. 32, 836–846 (2010).
Boele, F. W., Schilder, C. M., de Roode, M. L., Deijen, J. B. & Schagen, S. B. Cognitive functioning during long-term tamoxifen treatment in postmenopausal women with breast cancer. Menopause 22, 17–25 (2015).
Hermelink, K. et al. Short-term effects of treatment-induced hormonal changes on cognitive function in breast cancer patients: results of a multicenter, prospective, longitudinal study. Cancer 113, 2431–2439 (2008).
Breckenridge, L. M., Bruns, G. L., Todd, B. L. & Feuerstein, M. Cognitive limitations associated with tamoxifen and aromatase inhibitors in employed breast cancer survivors. Psychooncology 21, 43–53 (2012).
Bender, C. M. et al. Memory impairments with adjuvant anastrozole versus tamoxifen in women with early-stage breast cancer. Menopause 14, 995–998 (2007).
Ganz, P. A. et al. Cognitive function after the initiation of adjuvant endocrine therapy in early-stage breast cancer: an observational cohort study. J. Clin. Oncol. 32, 3559–3567 (2014).
Hurria, A. et al. The effect of aromatase inhibition on the cognitive function of older patients with breast cancer. Clin. Breast Cancer 14, 132–140 (2014).
Parker, J. S. et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 27, 1160–1167 (2009).
Drukker, C. A. et al. A prospective evaluation of a breast cancer prognosis signature in the observational RASTER study. Int. J. Cancer 133, 929–936 (2013).
Bogaerts, J. et al. Gene signature evaluation as a prognostic tool: challenges in the design of the MINDACT trial. Nat. Clin. Pract. Oncol. 3, 540–551 (2006).
Doyle, K. L. et al. Real-world impact of neurocognitive deficits in acute and early HIV infection. J. Neurovirol. 19, 565–573 (2013).
Tucker-Drob, E. M. Neurocognitive functions and everyday functions change together in old age. Neuropsychology 25, 368–377 (2011).
Dixon, J. M. et al. Anastrozole and letrozole: an investigation and comparison of quality of life and tolerability. Breast Cancer Res. Treat. 125, 741–749 (2011).
Fallowfield, L. J. et al. Long-term assessment of quality of life in the Intergroup Exemestane Study: 5 years post-randomisation. Br. J. Cancer 106, 1062–1067 (2012).
Tevaarwerk, A. J. et al. Phase III comparison of tamoxifen versus tamoxifen plus ovarian function suppression in premenopausal women with node-negative, hormone receptor-positive breast cancer (E3193, INT0142): a trial of the Eastern Cooperative Oncology Group. J. Clin. Oncol. 32, 3948–3958 (2014).
van den Bent, M. J. et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 12, 583–593 (2011).
Lin, N. U. et al. Challenges relating to solid tumour brain metastases in clinical trials, part 2: neurocognitive, neurological, and qualityoflife outcomes. A report from the RANO group. Lancet Oncol. 14, e407–e416 (2013).
Wefel, J. S., Vardy, J., Ahles, T. & Schagen, S. B. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 12, 703–708 (2011).
Acknowledgements
The authors thank Tesa Severson for critically reading the manuscript. The work of W.Z. is supported by an Alpe d'HuZes foundation/KWF Dutch Cancer Society Bas Mulder Award, A Sister's Hope and a Veni grant from The Netherlands Organization for Scientific Research (NWO). S.B.S. is supported by Dutch Cancer Society and Pink Ribbon grants, and S.C.L. by A Sister's Hope grant. S.C.L. is affiliated with the Department of Pathology, University Medical Center Utrecht.
Author information
Authors and Affiliations
Contributions
H.T. researched the data under supervision of W.Z. and W.Z. and S.B.S. wrote the manuscript with input from H.T. and S.C.L.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table
Overview of neuropsychological studies aimed to investigate the cognitive impact of endocrine therapies in breast cancer. (DOC 68 kb)
Rights and permissions
About this article
Cite this article
Zwart, W., Terra, H., Linn, S. et al. Cognitive effects of endocrine therapy for breast cancer: keep calm and carry on?. Nat Rev Clin Oncol 12, 597–606 (2015). https://doi.org/10.1038/nrclinonc.2015.124
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2015.124
This article is cited by
-
Multimodal MRI examination of structural and functional brain changes in older women with breast cancer in the first year of antiestrogen hormonal therapy
Breast Cancer Research and Treatment (2022)
-
Association of APOE4 genotype and treatment with cognitive outcomes in breast cancer survivors over time
npj Breast Cancer (2021)
-
Measuring decline in white matter integrity after systemic treatment for breast cancer: omitting skeletonization enhances sensitivity
Brain Imaging and Behavior (2021)
-
Self-reported cognitive decline in Japanese patients with breast cancer treated with endocrine therapy
Breast Cancer (2020)
-
Risk of dementia among postmenopausal breast cancer survivors treated with aromatase inhibitors versus tamoxifen: a cohort study using primary care data from the UK
Journal of Cancer Survivorship (2019)