Key Points
-
Neuroendocrine neoplasms (NENs) constitute a heterogeneous group of tumours that are each associated with varying clinical presentations, a different likelihood of progression, and a distinct prognosis
-
The 2010 WHO classification of NENs stratifies disease into NEN grade 1, NEN grade 2, neuroendocrine carcinoma (NEC) grade 3; this classification forms the basis of current treatment guidelines
-
Molecular profiling of tissue specimens is increasingly performed in clinical practice and provides information on genetic aberrations of potential therapeutic relevance; although with limited clinical value to date
-
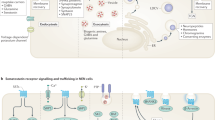
The 'driver' mutations in gastroenteropancreatic NENs, which might be important targets for new therapies, remain to be identified; however, the PI3K/AKT/mTOR axis has been identified as a key oncogenic signalling pathway
-
NENs can be treated using mTOR inhibitors (rapalogs including everolimus and temsirolimus), but resistance eventually develops via escape mechanisms that warrant further investigation in order to develop novel therapeutic strategies
-
The 2010 WHO classification will continue to guide the management of NENs; however, information from molecular profiling of tumours, 'liquid biopsies' (including circulating mRNA measurements), and molecular imaging might improve patient outcomes
Abstract
Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) constitute a heterogeneous group of tumours associated with variable clinical presentations, growth rates, and prognoses. To improve the management of GEP-NENs, the WHO developed a classification system that enables tumours to be graded based on markers of cell proliferation in biopsy specimens. Indeed, histopathology has been a mainstay in the diagnosis of GEP-NENs, and the WHO grading system facilitates therapeutic decision-making; however, considerable intratumoural heterogeneity, predominantly comprising regional variations in proliferation rates, complicates the evaluation of tumour biology. The use of molecular imaging modalities to delineate the most-aggressive cell populations is becoming more widespread. In addition, molecular profiling is increasingly undertaken in the clinical setting, and genomic studies have revealed a number of chromosomal alterations in GEP-NENs, although the 'drivers' of neoplastic development have not been identified. Thus, our molecular understanding of GEP-NENs remains insufficient to inform on patient prognosis or selection for treatments, and the WHO classification continues to form the basis for management of this disease. Nevertheless, our increasing understanding of the molecular genetics and biology of GEP-NENs has begun to expose flaws in the WHO classification. We describe the current understanding of the molecular characteristics of GEP-NENs, and discuss how advances in molecular profiling measurements, including assays of circulating mRNAs, are likely to influence the management of these tumours.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Modlin, I. M. et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 9, 61–72 (2008).
Yao, J. C. et al. One hundred years after 'carcinoid': epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 26, 3063–3072 (2008).
Lawrence, B. et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol. Metab. Clin. North Am. 40, 1–18 (2011).
de Mestier, L. et al. Evaluating digestive neuroendocrine tumor progression and therapeutic responses in the era of targeted therapies: state of the art. Endocr. Relat. Cancer 21, R105–R120 (2014).
Bergsland, E. K. The evolving landscape of neuroendocrine tumors. Semin. Oncol. 40, 4–22 (2013).
Modlin, I. M., Moss, S. F., Chung, D. C., Jensen, R. T. & Snyderwine, E. Priorities for improving the management of gastroenteropancreatic neuroendocrine tumors. J. Natl Cancer Inst. 100, 1282–1289 (2008).
Bosman, F. T. & Carneiro, F. WHO Classification of Tumours, Pathology and Genetics of Tumours of the Digestive System (IARC Press, 2010).
McCall, C. M. et al. Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. Am. J. Surg. Pathol. 37, 1671–1677 (2013).
Reid, M. D., Balci, S., Saka, B. & Adsay, N. V. Neuroendocrine tumors of the pancreas: current concepts and controversies. Endocr. Pathol. 25, 65–79 (2014).
Velayoudom-Cephise, F. L. et al. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocr. Relat. Cancer 20, 649–657 (2013).
The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012).
Jass, J. R. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 50, 113–130 (2007).
Domingo, E. et al. Use of multivariate analysis to suggest a new molecular classification of colorectal cancer. J. Pathol. 229, 441–448 (2013).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Schnitt, S. J. Classification and prognosis of invasive breast cancer: from morphology to molecular taxonomy. Mod. Pathol. 23, S60–S64 (2010).
Rindi, G. et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 449, 395–401 (2006).
Oberg, K. Neuroendocrine tumors of the digestive tract: impact of new classifications and new agents on therapeutic approaches. Curr. Opin. Oncol. 24, 433–440 (2012).
Sundin, A. Radiological and nuclear medicine imaging of gastroenteropancreatic neuroendocrine tumours. Best Pract. Res. Clin. Gastroenterol. 26, 803–818 (2012).
Baum, R. P., Kulkarni, H. R. & Carreras, C. Peptides and receptors in image-guided therapy: theranostics for neuroendocrine neoplasms. Semin. Nucl. Med. 42, 190–207 (2012).
Koopmans, K. P. et al. Staging of carcinoid tumours with 18F-DOPA PET: a prospective, diagnostic accuracy study. Lancet Oncol. 7, 728–734 (2006).
Kjaer, A. Molecular imaging of cancer using PET and SPECT. Adv. Exp. Med. Biol. 587, 277–284 (2006).
Binderup, T., Knigge, U., Loft, A., Federspiel, B. & Kjaer, A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin. Cancer Res. 16, 978–985 (2010).
Bahri, H. et al. High prognostic value of 18F-FDG PET for metastatic gastroenteropancreatic neuroendocrine tumors: a long-term evaluation. J. Nucl. Med. 55, 1786–1790 (2014).
Garin, E. & Edeline, J. Reply: modifying the poor prognosis associated with 18F-FDG-avid NET with peptide receptor chemo-radionuclide therapy (PRCRT). J. Nucl. Med. 56, 969 (2015).
Susini, C. & Buscail, L. Rationale for the use of somatostatin analogs as antitumor agents. Ann. Oncol. 17, 1733–1742 (2006).
Reubi, J. C. Somatostatin and other peptide receptors as tools for tumor diagnosis and treatment. Neuroendocrinology 80 (Suppl. 1), 51–56 (2004).
Bertherat, J. et al. Somatostatin receptors 2 and 5 are the major somatostatin receptors in insulinomas: an in vivo and in vitro study. J. Clin. Endocrinol. Metab. 88, 5353–5360 (2003).
Mirallie, E. et al. Value of endoscopic ultrasonography and somatostatin receptor scintigraphy in the preoperative localization of insulinomas and gastrinomas. Experience of 54 cases. Gastroenterol. Clin. Biol. 26, 360–366 (in French) (2002).
Vezzosi, D. et al. Octreotide in insulinoma patients: efficacy on hypoglycemia, relationships with Octreoscan scintigraphy and immunostaining with anti-sst2A and anti-sst5 antibodies. Eur. J. Endocrinol. 152, 757–767 (2005).
Srirajaskanthan, R., Watkins, J., Marelli, L., Khan, K. & Caplin, M. E. Expression of somatostatin and dopamine 2 receptors in neuroendocrine tumours and the potential role for new biotherapies. Neuroendocrinology 89, 308–314 (2009).
Kvols, L. K. et al. The presence of somatostatin receptors in malignant neuroendocrine tumor tissue predicts responsiveness to octreotide. Yale J. Biol. Med. 65, 505–518; discussion 531–536 (1992).
Rinke, A. et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J. Clin. Oncol. 27, 4656–4663 (2009).
Caplin, M. E. et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 371, 224–233 (2014).
Kvols, L. K. et al. Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: results from a phase II study. Endocr. Relat. Cancer 19, 657–666 (2012).
Lebtahi, R. et al. Clinical impact of somatostatin receptor scintigraphy in the management of patients with neuroendocrine gastroenteropancreatic tumors. J. Nucl. Med. 38, 853–858 (1997).
Bodei, L., Sundin, A., Kidd, M., Prasad, V. & Modlin, I. M. The status of neuroendocrine tumor imaging: from darkness to light? Neuroendocrinology 101, 1–17 (2015).
Ruszniewski, P. ECC 2015 press release: rare cancer responds unusually well to new treatment: results from the NETTER-1 trial. ESMO http://www.esmo.org/Conferences/Past-Conferences/European-Cancer-Congress-2015/News/Rare-cancer-responds-unusually-well-to-new-treatment-results-from-the-NETTER-1-trial (2015).
Okuwaki, K. et al. Clinicopathologic characteristics of pancreatic neuroendocrine tumors and relation of somatostatin receptor type 2A to outcomes. Cancer 119, 4094–4102 (2013).
Pinato, D. J. et al. An expression signature of the angiogenic response in gastrointestinal neuroendocrine tumours: correlation with tumour phenotype and survival outcomes. Br. J. Cancer 110, 115–122 (2014).
Campana, D. et al. Standardized uptake values of 68Ga-DOTANOC PET: a promising prognostic tool in neuroendocrine tumors. J. Nucl. Med. 51, 353–359 (2010).
Kwekkeboom, D. J. et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr. Relat. Cancer 17, R53–R73 (2010).
Kidd, M., Modlin, I. M., Bodei, L. & Drozdov, I. Decoding the molecular and mutational ambiguities of gastroenteropancreatic neuroendocrine neoplasm pathobiology. Cell. Mol. Gastroenterol. Hepatol. 1, 131–153 (2015).
Larsson, C., Skogseid, B., Oberg, K., Nakamura, Y. & Nordenskjold, M. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature 332, 85–87 (1988).
Hughes, C. M. et al. Menin associates with a trithorax family histone methyltransferase complex and with the hoxc8 locus. Mol. Cell 13, 587–597 (2004).
Richard, S. et al. Von Hippel–Lindau disease: recent genetic progress and patient management. Francophone Study Group of von Hippel–Lindau Disease (GEFVH). Ann. Endocrinol. (Paris) 59, 452–458 (in French) (1998).
Ruggieri, M. & Huson, S. M. The neurofibromatoses. An overview. Ital. J. Neurol. Sci. 20, 89–108 (1999).
Au, K. S., Williams, A. T., Gambello, M. J. & Northrup, H. Molecular genetic basis of tuberous sclerosis complex: from bench to bedside. J. Child Neurol. 19, 699–709 (2004).
Matkar, S., Thiel, A. & Hua, X. Menin: a scaffold protein that controls gene expression and cell signaling. Trends Biochem. Sci. 38, 394–402 (2013).
Bluyssen, H. A. et al. Fibronectin is a hypoxia-independent target of the tumor suppressor VHL. FEBS Lett. 556, 137–142 (2004).
Kibel, A., Iliopoulos, O., DeCaprio, J. A. & Kaelin, W. G. Jr. Binding of the von Hippel–Lindau tumor suppressor protein to Elongin B and C. Science 269, 1444–1446 (1995).
Faggiano, A. et al. Natural history of gastro-entero-pancreatic and thoracic neuroendocrine tumors. Data from a large prospective and retrospective Italian epidemiological study: the NET management study. J. Endocrinol. Invest. 35, 817–823 (2012).
Jiao, Y. et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 331, 1199–1203 (2011).
Langer, P. et al. Prospective evaluation of imaging procedures for the detection of pancreaticoduodenal endocrine tumors in patients with multiple endocrine neoplasia type 1. World J. Surg. 28, 1317–1322 (2004).
van Asselt, S. J. et al. EUS is superior for detection of pancreatic lesions compared with standard imaging in patients with multiple endocrine neoplasia type 1. Gastrointest. Endosc. 81, 159–167. e2 (2015).
de Wilde, R. F. et al. Loss of ATRX or DAXX expression and concomitant acquisition of the alternative lengthening of telomeres phenotype are late events in a small subset of MEN-1 syndrome pancreatic neuroendocrine tumors. Mod. Pathol. 25, 1033–1039 (2012).
Corcos, O. et al. Endocrine pancreatic tumors in von Hippel–Lindau disease: clinical, histological, and genetic features. Pancreas 37, 85–93 (2008).
Sei, Y. et al. A hereditary form of small intestinal carcinoid associated with a germline mutation in inositol polyphosphate multikinase. Gastroenterology 149, 67–78 (2015).
Spurdle, A. B. et al. Refined histopathological predictors of BRCA1 and BRCA2 mutation status: a large-scale analysis of breast cancer characteristics from the BCAC, CIMBA, and ENIGMA consortia. Breast Cancer Res. 16, 3419 (2014).
Jonkers, Y. M. et al. Chromosomal instability predicts metastatic disease in patients with insulinomas. Endocr. Relat. Cancer 12, 435–447 (2005).
Zhuang, Z. et al. Somatic mutations of the MEN1 tumor suppressor gene in sporadic gastrinomas and insulinomas. Cancer Res. 57, 4682–4686 (1997).
Cao, Y. et al. Whole exome sequencing of insulinoma reveals recurrent T372R mutations in YY1. Nat. Commun. 4, 2810 (2013).
Shay, J. W., Reddel, R. R. & Wright, W. E. Cancer and telomeres — an ALTernative to telomerase. Science 336, 1388–1390 (2012).
Heaphy, C. M. et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science 333, 425 (2011).
Marinoni, I. et al. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology 146, 453–460. e5 (2014).
Zhou, C., Dhall, D., Nissen, N. N., Chen, C. R. & Yu, R. Homozygous P86S mutation of the human glucagon receptor is associated with hyperglucagonemia, α cell hyperplasia, and islet cell tumor. Pancreas 38, 941–946 (2009).
Lin, W. et al. Dynamic epigenetic regulation by menin during pancreatic islet tumor formation. Mol. Cancer Res. 13, 689–698 (2015).
Gurung, B. et al. PTCH 1 staining of pancreatic neuroendocrine tumor (PNET) samples from patients with and without multiple endocrine neoplasia (MEN-1) syndrome reveals a potential therapeutic target. Cancer Biol. Ther. 16, 219–224 (2015).
Banck, M. S. et al. The genomic landscape of small intestine neuroendocrine tumors. J. Clin. Invest. 123, 2502–2508 (2013).
Francis, J. M. et al. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat. Genet. 45, 1483–1486 (2013).
Lee, M. & Pellegata, N. S. Multiple endocrine neoplasia syndromes associated with mutation of p27. J. Endocrinol. Invest. 36, 781–787 (2013).
Kytola, S. et al. Alterations of the SDHD gene locus in midgut carcinoids, Merkel cell carcinomas, pheochromocytomas, and abdominal paragangliomas. Genes Chromosomes Cancer 34, 325–332 (2002).
Karnik, S. K. et al. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc. Natl Acad. Sci. USA 102, 14659–14664 (2005).
Ghimenti, C., Lonobile, A., Campani, D., Bevilacqua, G. & Caligo, M. A. Microsatellite instability and allelic losses in neuroendocrine tumors of the gastro-entero-pancreatic system. Int. J. Oncol. 15, 361–366 (1999).
Arnold, C. N., Sosnowski, A. & Blum, H. E. Analysis of molecular pathways in neuroendocrine cancers of the gastroenteropancreatic system. Ann. NY Acad. Sci. 1014, 218–219 (2004).
House, M. G. et al. Prognostic value of hMLH1 methylation and microsatellite instability in pancreatic endocrine neoplasms. Surgery 134, 902–908; discussion 909 (2003).
Kidd, M. et al. Microsatellite instability and gene mutations in transforming growth factor-beta type II receptor are absent in small bowel carcinoid tumors. Cancer 103, 229–236 (2005).
Speel, E. J. et al. Genetic differences in endocrine pancreatic tumor subtypes detected by comparative genomic hybridization. Am. J. Pathol. 155, 1787–1794 (1999).
Speel, E. J. et al. Genetic evidence for early divergence of small functioning and nonfunctioning endocrine pancreatic tumors: gain of 9Q34 is an early event in insulinomas. Cancer Res. 61, 5186–5192 (2001).
Simon, B. & Lubomierski, N. Implication of the INK4a/ARF locus in gastroenteropancreatic neuroendocrine tumorigenesis. Ann. NY Acad. Sci. 1014, 284–299 (2004).
Zikusoka, M. N., Kidd, M., Eick, G., Latich, I. & Modlin, I. M. The molecular genetics of gastroenteropancreatic neuroendocrine tumors. Cancer 104, 2292–2309 (2005).
Perren, A. et al. Mutation and expression analyses reveal differential subcellular compartmentalization of PTEN in endocrine pancreatic tumors compared to normal islet cells. Am. J. Pathol. 157, 1097–1103 (2000).
Missiaglia, E. et al. Pancreatic endocrine tumors: expression profiling evidences a role for AKT–mTOR pathway. J. Clin. Oncol. 28, 245–255 (2010).
Hu, W. et al. Gene amplifications in well-differentiated pancreatic neuroendocrine tumors inactivate the p53 pathway. Genes Cancer 1, 360–368 (2010).
Kytola, S. et al. Comparative genomic hybridization identifies loss of 18q22-qter as an early and specific event in tumorigenesis of midgut carcinoids. Am. J. Pathol. 158, 1803–1808 (2001).
Lollgen, R. M., Hessman, O., Szabo, E., Westin, G. & Akerstrom, G. Chromosome 18 deletions are common events in classical midgut carcinoid tumors. Int. J. Cancer 92, 812–815 (2001).
Andersson, E., Sward, C., Stenman, G., Ahlman, H. & Nilsson, O. High-resolution genomic profiling reveals gain of chromosome 14 as a predictor of poor outcome in ileal carcinoids. Endocr. Relat. Cancer 16, 953–966 (2009).
Arnold, C. N., Sosnowski, A., Schmitt-Graff, A., Arnold, R. & Blum, H. E. Analysis of molecular pathways in sporadic neuroendocrine tumors of the gastro-entero-pancreatic system. Int. J. Cancer 120, 2157–2164 (2007).
Chan, A. O. et al. CpG island methylation in carcinoid and pancreatic endocrine tumors. Oncogene 22, 924–934 (2003).
House, M. G. et al. Aberrant hypermethylation of tumor suppressor genes in pancreatic endocrine neoplasms. Ann. Surg. 238, 423–431; discussion 431–432 (2003).
Kulke, M. H. et al. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin. Cancer Res. 15, 338–345 (2009).
Walter, T. et al. O6-methylguanine-DNA methyltransferase status in neuroendocrine tumours: prognostic relevance and association with response to alkylating agents. Br. J. Cancer 112, 523–531 (2015).
Schmitt, A. M. et al. VHL inactivation is an important pathway for the development of malignant sporadic pancreatic endocrine tumors. Endocr. Relat. Cancer 16, 1219–1227 (2009).
Dejeux, E. et al. Hypermethylation of the IGF2 differentially methylated region 2 is a specific event in insulinomas leading to loss-of-imprinting and overexpression. Endocr. Relat. Cancer 16, 939–952 (2009).
Boissan, M. et al. Implication of metastasis suppressor NM23-H1 in maintaining adherens junctions and limiting the invasive potential of human cancer cells. Cancer Res. 70, 7710–7722 (2010).
Hendy, G. N., Kaji, H., Sowa, H., Lebrun, J. J. & Canaff, L. Menin and TGF-β superfamily member signaling via the Smad pathway in pituitary, parathyroid and osteoblast. Horm. Metab. Res. 37, 375–379 (2005).
Gurung, B., Feng, Z. & Hua, X. Menin directly represses Gli1 expression independent of canonical Hedgehog signaling. Mol. Cancer Res. 11, 1215–1222 (2013).
Yang, Y. J. et al. Menin mediates epigenetic regulation via histone H3 lysine 9 methylation. Cell Death Dis. 4, e583 (2013).
Fotouhi, O. et al. Global hypomethylation and promoter methylation in small intestinal neuroendocrine tumors: an in vivo and in vitro study. Epigenetics 9, 987–997 (2014).
Zhang, H. Y. et al. Association of DNA methylation and epigenetic inactivation of RASSF1A and beta-catenin with metastasis in small bowel carcinoid tumors. Endocrine 30, 299–306 (2006).
Duerr, E. M. et al. Defining molecular classifications and targets in gastroenteropancreatic neuroendocrine tumors through DNA microarray analysis. Endocr. Relat. Cancer 15, 243–256 (2008).
Dilley, W. G. et al. Global gene expression in neuroendocrine tumors from patients with the MEN1 syndrome. Mol. Cancer 4, 9 (2005).
Roldo, C. et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J. Clin. Oncol. 24, 4677–4684 (2006).
Kidd, M., Modlin, I. M. & Drozdov, I. Gene network-based analysis identifies two potential subtypes of small intestinal neuroendocrine tumors. BMC Genomics 15, 595 (2014).
Kidd, M. et al. The role of genetic markers — NAP1L1, MAGE-D2, and MTA1 — in defining small-intestinal carcinoid neoplasia. Ann. Surg. Oncol. 13, 253–262 (2006).
Leja, J. et al. Novel markers for enterochromaffin cells and gastrointestinal neuroendocrine carcinomas. Mod. Pathol. 22, 261–272 (2009).
Cui, T. et al. Paraneoplastic antigen Ma2 autoantibodies as specific blood biomarkers for detection of early recurrence of small intestine neuroendocrine tumors. PLoS ONE 5, e16010 (2010).
Li, S. C. et al. Global microRNA profiling of well-differentiated small intestinal neuroendocrine tumors. Mod. Pathol. 26, 685–696 (2013).
Kerr, S. E. et al. A 92-gene cancer classifier predicts the site of origin for neuroendocrine tumors. Mod. Pathol. 27, 44–54 (2014).
Modlin, I. M., Kidd, M., Bodei, L., Drozdov, I. & Aslanian, H. The clinical utility of a novel blood-based multi-transcriptome assay for the diagnosis of neuroendocrine tumors of the gastrointestinal tract. Am. J. Gastroenterol. 110, 1223–1232 (2015).
Kidd, M., Drozdov, I. & Modlin, I. Blood and tissue neuroendocrine tumor gene cluster analysis correlate, define hallmarks and predict disease status. Endocr. Relat. Cancer 22, 561–575 (2015).
Svejda, B. et al. Limitations in small intestinal neuroendocrine tumor therapy by mTor kinase inhibition reflect growth factor-mediated PI3K feedback loop activation via ERK1/2 and AKT. Cancer 117, 4141–4154 (2011).
Li, H. J., Kapoor, A., Giel-Moloney, M., Rindi, G. & Leiter, A. B. Notch signaling differentially regulates the cell fate of early endocrine precursor cells and their maturing descendants in the mouse pancreas and intestine. Dev. Biol. 371, 156–169 (2012).
Fendrich, V. et al. Snail and Sonic Hedgehog activation in neuroendocrine tumors of the ileum. Endocr. Relat. Cancer 14, 865–874 (2007).
Shida, T. et al. Sonic Hedgehog–Gli1 signaling pathway might become an effective therapeutic target in gastrointestinal neuroendocrine carcinomas. Cancer Biol. Ther. 5, 1530–1538 (2006).
Di Florio, A. et al. Src kinase activity coordinates cell adhesion and spreading with activation of mammalian target of rapamycin in pancreatic endocrine tumour cells. Endocr. Relat. Cancer 18, 541–554 (2011).
von Wichert, G. et al. Insulin-like growth factor-I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Res. 60, 4573–4581 (2000).
Besig, S., Voland, P., Baur, D. M., Perren, A. & Prinz, C. Vascular endothelial growth factors, angiogenesis, and survival in human ileal enterochromaffin cell carcinoids. Neuroendocrinology 90, 402–415 (2009).
Wolin, E. M. The expanding role of somatostatin analogs in the management of neuroendocrine tumors. Gastrointest. Cancer Res. 5, 161–168 (2012).
Oberg, K. et al. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann. Oncol. 15, 966–973 (2004).
Shah, T. et al. Epidermal growth factor receptor expression and activation in neuroendocrine tumours. J. Neuroendocrinol. 18, 355–360 (2006).
Very, N. M., Kittilson, J. D., Klein, S. E. & Sheridan, M. A. Somatostatin inhibits basal and growth hormone-stimulated hepatic insulin-like growth factor-I production. Mol. Cell. Endocrinol. 281, 19–26 (2008).
Svejda, B. et al. Serotonin and the 5-HT7 receptor: a link between hepatocytes, IGF-1 and small intestinal neuroendocrine tumors. Cancer Sci. 104, 844–855 (2013).
Porta, C., Paglino, C. & Mosca, A. Targeting PI3K/Akt/mTOR signaling in Cancer. Front. Oncol. 4, 64 (2014).
Yang, Q. & Guan, K. L. Expanding mTOR signaling. Cell Res. 17, 666–681 (2007).
Kasajima, A. et al. mTOR expression and activity patterns in gastroenteropancreatic neuroendocrine tumours. Endocr. Relat. Cancer 18, 181–192 (2011).
Larson, A. M. et al. Pancreatic neuroendocrine tumors in patients with tuberous sclerosis complex. Clin. Genet. 82, 558–563 (2012).
Han, X., Ji, Y., Zhao, J., Xu, X. & Lou, W. Expression of PTEN and mTOR in pancreatic neuroendocrine tumors. Tumour Biol. 34, 2871–2879 (2013).
Wang, L., Ignat, A. & Axiotis, C. A. Differential expression of the PTEN tumor suppressor protein in fetal and adult neuroendocrine tissues and tumors: progressive loss of PTEN expression in poorly differentiated neuroendocrine neoplasms. Appl. Immunohistochem. Mol. Morphol. 10, 139–146 (2002).
O'Toole, D. et al. Molecular markers associated with response to chemotherapy in gastro-entero-pancreatic neuroendocrine tumors. Endocr. Relat. Cancer 17, 847–856 (2010).
Yao, J. C. et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 364, 514–523 (2011).
Pavel, M. E. et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 378, 2005–2012 (2011).
Yao, J. C. et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 387, 968–977 (2016).
Zitzmann, K. et al. Compensatory activation of Akt in response to mTOR and Raf inhibitors — a rationale for dual-targeted therapy approaches in neuroendocrine tumor disease. Cancer Lett. 295, 100–109 (2010).
Moelling, K., Schad, K., Bosse, M., Zimmermann, S. & Schweneker, M. Regulation of Raf–Akt cross-talk. J. Biol. Chem. 277, 31099–31106 (2002).
Zhu, J., Blenis, J. & Yuan, J. Activation of PI3K/Akt and MAPK pathways regulates Myc-mediated transcription by phosphorylating and promoting the degradation of Mad1. Proc. Natl Acad. Sci. USA 105, 6584–6589 (2008).
Reusch, H. P., Zimmermann, S., Schaefer, M., Paul, M. & Moelling, K. Regulation of Raf by Akt controls growth and differentiation in vascular smooth muscle cells. J. Biol. Chem. 276, 33630–33637 (2001).
Rozengurt, E., Soares, H. P. & Sinnet-Smith, J. Suppression of feedback loops mediated by PI3K/mTOR induces multiple overactivation of compensatory pathways: an unintended consequence leading to drug resistance. Mol. Cancer Ther. 13, 2477–2488 (2014).
US National Library of Science. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01628913 (2016).
Salazar, R. et al. Phase II studies of BEZ235 in patients with advanced pancreatic neuroendocrine tumors (pNET) [abstract]. J. Clin. Oncol. 33 (Suppl.), 4102 (2015).
Perren, A. et al. BRAF and endocrine tumors: mutations are frequent in papillary thyroid carcinomas, rare in endocrine tumors of the gastrointestinal tract and not detected in other endocrine tumors. Endocr. Relat. Cancer 11, 855–860 (2004).
Karhoff, D. et al. Rap1/B-Raf signaling is activated in neuroendocrine tumors of the digestive tract and Raf kinase inhibition constitutes a putative therapeutic target. Neuroendocrinology 85, 45–53 (2007).
Wulbrand, U. et al. Growth factor receptor expression in human gastroenteropancreatic neuroendocrine tumours. Eur. J. Clin. Invest. 28, 1038–1049 (1998).
Nilsson, O., Wangberg, B., Theodorsson, E., Skottner, A. & Ahlman, H. Presence of IGF-I in human midgut carcinoid tumours — an autocrine regulator of carcinoid tumour growth? Int. J. Cancer 51, 195–203 (1992).
Samani, A. A., Yakar, S., LeRoith, D. & Brodt, P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr. Rev. 28, 20–47 (2007).
Gloesenkamp, C. et al. Heat shock protein 90 is a promising target for effective growth inhibition of gastrointestinal neuroendocrine tumors. Int. J. Oncol. 40, 1659–1667 (2012).
Reidy-Lagunes, D. L. et al. A phase 2 study of the insulin-like growth factor-1 receptor inhibitor MK-0646 in patients with metastatic, well-differentiated neuroendocrine tumors. Cancer 118, 4795–4800 (2012).
Strosberg, J. R. et al. A multi-institutional, phase II open-label study of ganitumab (AMG 479) in advanced carcinoid and pancreatic neuroendocrine tumors. Endocr. Relat. Cancer 20, 383–390 (2013).
Libutti, S. K. Therapy: blockade of IGF-1R-not effective in neuroendocrine tumours. Nat. Rev. Endocrinol. 9, 389–390 (2013).
US National Library of Science. ClinicalTrials.gov [online], https://clinicaltrials.gov/ct2/show/NCT00781911 (2016).
US National Library of Science. ClinicalTrials.gov [online], https://clinicaltrials.gov/ct2/show/NCT01431547 (2015).
Gilbert, J. A. et al. Molecular markers for novel therapies in neuroendocrine (carcinoid) tumors. Endocr. Relat. Cancer 17, 623–636 (2010).
US National Library of Science. ClinicalTrials.gov [online], https://clinicaltrials.gov/ct2/show/NCT00843531 (2015).
US National Library of Science. ClinicalTrials.gov [online], https://clinicaltrials.gov/ct2/show/NCT01172717 (2013).
Olsson, A. K., Dimberg, A., Kreuger, J. & Claesson-Welsh, L. VEGF receptor signalling — in control of vascular function. Nat. Rev. Mol. Cell Biol. 7, 359–371 (2006).
Bowen, K. A. et al. An analysis of trends and growth factor receptor expression of GI carcinoid tumors. J. Gastrointest. Surg. 13, 1773–1780 (2009).
Kidd, M. et al. CTGF, intestinal stellate cells and carcinoid fibrogenesis. World J. Gastroenterol. 13, 5208–5216 (2007).
Zhang, J. et al. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer 109, 1478–1486 (2007).
Yao, J. C. et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alfa-2b. J. Clin. Oncol. 26, 1316–1323 (2008).
Chan, J. A. et al. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J. Clin. Oncol. 30, 2963–2968 (2012).
Castellano, D. et al. Sorafenib and bevacizumab combination targeted therapy in advanced neuroendocrine tumor: a phase II study of the Spanish Neuroendocrine Tumors Group (GETNE0801). J. Clin. Oncol. 49, 3780–3787 (2013).
Phan, A. T. et al. Pazopanib and depot octreotide in advanced, well-differentiated neuroendocrine tumours: a multicentre, single-group, phase 2 study. Lancet Oncol. 16, 695–703 (2015).
Raymond, E. et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 364, 501–513 (2011).
Zurita, A. J. et al. Circulating cytokines and monocyte subpopulations as biomarkers of outcome and biological activity in sunitinib-treated patients with advanced neuroendocrine tumours. Br. J. Cancer 112, 1199–1205 (2015).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Khan, M. S. et al. Circulating tumor cells as prognostic markers in neuroendocrine tumors. J. Clin. Oncol. 31, 365–372 (2013).
Modlin, I. M. et al. A multianalyte PCR blood test outperforms single analyte ELISAs (chromogranin A, pancreastatin, neurokinin A) for neuroendocrine tumor detection. Endocr. Relat. Cancer 21, 615–628 (2014).
Modlin, I. M., Drozdov, I. & Kidd, M. The identification of gut neuroendocrine tumor disease by multiple synchronous transcript analysis in blood. PLoS ONE 8, e63364 (2013).
US National Library of Science. ClinicalTrials.gov [online], https://clinicaltrials.gov/ct2/show/NCT00428220 (2015).
US National Library of Science. ClinicalTrials.gov [online], https://clinicaltrials.gov/ct2/show/NCT02315625 (2016).
Acknowledgements
The authors thank Xia Chu of the University of Uppsala, Sweden, for his assistance in developing Fig. 2.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
I.M. is a consultant to Wren Laboratories, a subsidiary of Clifton Life Sciences. M.K. and K.Ö. declare no competing interests.
Rights and permissions
About this article
Cite this article
Kidd, M., Modlin, I. & Öberg, K. Towards a new classification of gastroenteropancreatic neuroendocrine neoplasms. Nat Rev Clin Oncol 13, 691–705 (2016). https://doi.org/10.1038/nrclinonc.2016.85
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2016.85
This article is cited by
-
Resection of the primary tumor improves the prognosis of gastrointestinal neuroendocrine neoplasms with liver metastases: mutual validation based on SEER database and institutional data
BMC Gastroenterology (2023)
-
Imaging and liquid biopsy in the prediction and evaluation of response to PRRT in neuroendocrine tumors: implications for patient management
European Journal of Nuclear Medicine and Molecular Imaging (2021)
-
Neuroendocrine neoplasia of the gastrointestinal tract revisited: towards precision medicine
Nature Reviews Endocrinology (2020)
-
Modified AJCC staging of gastric neuroendocrine carcinoma based on T staging can improve the capacity of prognosis assessment
Journal of Cancer Research and Clinical Oncology (2018)
-
Biology and evolution of poorly differentiated neuroendocrine tumors
Nature Medicine (2017)