Key Points
-
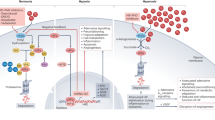
This Review highlights the therapeutic potential of inhibitors of a novel class of oxygen sensors — prolyl hydroxylase domain-containing proteins (PHDs) and factor inhibiting HIF (FIH) — to treat inflammatory and ischaemic diseases.
-
Oxygen sensors destabilize hypoxia inducible factors (HIFs), which are molecules that execute the cellular response to hypoxia. Induction of this HIF programme is associated with enhanced angiogenesis. Inhibition of PHDs might therefore offer opportunities for therapeutic revascularization of ischaemic tissues.
-
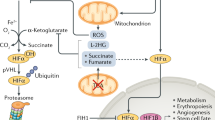
Inhibition of PHD1 results in ischaemia tolerance of skeletal muscle and other tissues; this protection is attributed to an attenuation of oxidative damage.
-
Inhibition of PHDs bears resemblance to changes in metabolism observed in hibernating species. This raises the possibility of therapeutically inducing (via inhibiting PHDs) a state of hibernation in various organs, which would confer cytoprotection against transient ischaemic periods during surgical (for example, bypass operations) or transplantation procedures.
-
Ischaemic preconditioning (IPC), a stressful but non-damaging hypoxic stimulus, confers protection against a subsequent harmful ischaemic event. Recent data indicate that inhibition of PHDs can induce IPC in vivo and confer resistance to hypoxic events in cardiac and cerebral tissues.
-
Inhibition of PHDs is also cytoprotective during development of inflammatory bowel disease in a murine model of colitis.
-
Most currently available PHD/FIH inhibitors are non-selective. Therefore, there is a need to develop novel, more specific inhibitors for each of the PHD isoforms. We discuss recently developed PHD inhibitors, and also introduce alternative approaches to generate more selective PHD inhibitors.
Abstract
Cells in the human body need oxygen to function and survive, and severe deprivation of oxygen, as occurs in ischaemic heart disease and stroke, is a major cause of mortality. Nevertheless, other organisms, such as the fossorial mole rat or diving seals, have acquired the ability to survive in conditions of limited oxygen supply. Hypoxia tolerance also allows the heart to survive chronic oxygen shortage, and ischaemic preconditioning protects tissues against lethal hypoxia. The recent discovery of a new family of oxygen sensors — including prolyl hydroxylase domain-containing proteins 1–3 (PHD1–3) — has yielded exciting novel insights into how cells sense oxygen and keep oxygen supply and consumption in balance. Advances in understanding of the role of these oxygen sensors in hypoxia tolerance, ischaemic preconditioning and inflammation are creating new opportunities for pharmacological interventions for ischaemic and inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Semenza, G. L. Targeting HIF-1 for cancer therapy. Nature Rev. Cancer 3, 721–732 (2003).
Wang, G. L., Jiang, B. H., Rue, E. A. & Semenza, G. L. Hypoxia-inducible factor 1 is a basic-helix–loop–helix–PAS heterodimer regulated by cellular O2 tension. Proc. Natl Acad. Sci. USA 92, 5510–5514 (1995).
Kaelin, W. G. Jr, & Ratcliffe, P. J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 (2008).
Bruick, R. K. & McKnight, S. L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294, 1337–1340 (2001).
Epstein, A. C. et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 (2001). References 4 and 5 describe the initial identification and characterization of HIF-regulating PHDs.
Lando, D. et al. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 16, 1466–1471 (2002).
Mahon, P. C., Hirota, K. & Semenza, G. L. FIH-1: a novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 15, 2675–2686 (2001).
Ehrismann, D. et al. Studies on the activity of the hypoxia-inducible-factor hydroxylases using an oxygen consumption assay. Biochem. J. 401, 227–234 (2007).
Koivunen, P., Hirsila, M., Gunzler, V., Kivirikko, K. I. & Myllyharju, J. Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. J. Biol. Chem. 279, 9899–9904 (2004).
Gnaiger, E., Lassnig, B., Kuznetsov, A., Rieger, G. & Margreiter, R. Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J. Exp. Biol. 201, 1129–1139 (1998).
Ward, J. P. Oxygen sensors in context. Biochim. Biophys. Acta 1777, 1–14 (2008).
Jaakkola, P. et al. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001).
Ivan, M. et al. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 (2001).
Stolze, I. P. et al. Genetic analysis of the role of the asparaginyl hydroxylase factor inhibiting hypoxia-inducible factor (HIF) in regulating HIF transcriptional target genes. J. Biol. Chem. 279, 42719–42725 (2004).
Ramirez, J. M., Folkow, L. P. & Blix, A. S. Hypoxia tolerance in mammals and birds: from the wilderness to the clinic. Annu. Rev. Physiol. 69, 113–143 (2007).
Kelly, D. P. Hypoxic reprogramming. Nature Genet. 40, 132–134 (2008).
Schmitz, A. & Harrison, J. F. Hypoxic tolerance in air-breathing invertebrates. Respir. Physiol. Neurobiol. 141, 229–242 (2004).
Aragones, J. et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nature Genet. 40, 170–180 (2008). This study provides in vivo evidence that therapeutic inhibition of PHD1 results in protection of skeletal muscle fibres against an otherwise lethal ischaemic insult via reprogramming of basal metabolism and subsequent attenuation of oxidative stress.
Eckle, T., Kohler, D., Lehmann, R., El Kasmi, K. & Eltzschig, H. K. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation 118, 166–175 (2008). This study identifies cardiac PHD2 as an essential player in cardiac protection against ischaemia–reperfusion injury via the adenosine pathway.
Simons, M. Angiogenesis: where do we stand now? Circulation 111, 1556–1566 (2005).
Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nature Med. 6, 389–395 (2000).
Harris, A. L. Hypoxia — a key regulatory factor in tumour growth. Nature Rev. Cancer 2, 38–47 (2002).
Pugh, C. W. & Ratcliffe, P. J. Regulation of angiogenesis by hypoxia: role of the HIF system. Nature Med. 9, 677–684 (2003).
Shyu, K. G. et al. Intramyocardial injection of naked DNA encoding HIF-1α/VP16 hybrid to enhance angiogenesis in an acute myocardial infarction model in the rat. Cardiovasc. Res. 54, 576–583 (2002).
Vincent, K. A. et al. Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding an HIF-1α/VP16 hybrid transcription factor. Circulation 102, 2255–2261 (2000). This paper confirms the utility of increasing HIF1α expression in therapeutic angiogenesis.
Kido, M. et al. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J. Am. Coll. Cardiol. 46, 2116–2124 (2005).
Takeda, K., Cowan, A. & Fong, G. H. Essential role for prolyl hydroxylase domain protein 2 in oxygen homeostasis of the adult vascular system. Circulation 116, 774–781 (2007).
Minamishima, Y. A. et al. Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure. Blood 111, 3236–3244 (2008).
Takeda, K. & Fong, G. H. Prolyl hydroxylase domain 2 protein suppresses hypoxia-induced endothelial cell proliferation. Hypertension 49, 178–184 (2007).
Knowles, H. J., Tian, Y. M., Mole, D. R. & Harris, A. L. Novel mechanism of action for hydralazine: induction of hypoxia-inducible factor-1α, vascular endothelial growth factor, and angiogenesis by inhibition of prolyl hydroxylases. Circ. Res. 95, 162–169 (2004).
Milkiewicz, M., Pugh, C. W. & Egginton, S. Inhibition of endogenous HIF inactivation induces angiogenesis in ischaemic skeletal muscles of mice. J. Physiol. 560, 21–26 (2004).
Campbell, W. B., Graham, R. M., Jackson, E. K., Loisel, D. P. & Pettinger, W. A. Effect of indomethacin on hydralazine-induced renin and catecholamine release in the conscious rabbit. Br. J. Pharmacol. 71, 529–531 (1980).
Amaral, S. L., Roman, R. J. & Greene, A. S. Renin gene transfer restores angiogenesis and vascular endothelial growth factor expression in Dahl S rats. Hypertension 37, 386–390 (2001).
Freret, T. et al. Delayed administration of deferoxamine reduces brain damage and promotes functional recovery after transient focal cerebral ischemia in the rat. Eur. J. Neurosci. 23, 1757–1765 (2006).
Siddiq, A. et al. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system. J. Biol. Chem. 280, 41732–41743 (2005). This paper illustrates the use of PHD inhibitors to confer neuroprotection in a mouse model of permanent cerebral focal ischaemia.
Hayashi, T., Abe, K. & Itoyama, Y. Reduction of ischemic damage by application of vascular endothelial growth factor in rat brain after transient ischemia. J. Cereb. Blood Flow Metab. 18, 887–895 (1998).
Manoonkitiwongsa, P. S., Schultz, R. L., McCreery, D. B., Whitter, E. F. & Lyden, P. D. Neuroprotection of ischemic brain by vascular endothelial growth factor is critically dependent on proper dosage and may be compromised by angiogenesis. J. Cereb. Blood Flow Metab. 24, 693–702 (2004).
Shen, F. et al. Adeno-associated viral-vector-mediated hypoxia-inducible vascular endothelial growth factor gene expression attenuates ischemic brain injury after focal cerebral ischemia in mice. Stroke 37, 2601–2606 (2006).
Gatenby, R. A. & Gillies, R. J. Why do cancers have high aerobic glycolysis? Nature Rev. Cancer 4, 891–899 (2004).
Comerford, K. M. et al. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 62, 3387–3394 (2002).
Zhang, H. et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 11, 407–420 (2007).
Zhang, H. et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 283, 10892–10903 (2008).
Dahia, P. L. et al. A HIF1α regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 1, 72–80 (2005).
Fukuda, R. et al. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129, 111–122 (2007).
Kim, J. W., Tchernyshyov, I., Semenza, G. L. & Dang, C. V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell. Metab. 3, 177–185 (2006).
Papandreou, I., Cairns, R. A., Fontana, L., Lim, A. L. & Denko, N. C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell. Metab. 3, 187–197 (2006). References 45 and 46 identified PDK1 as a HIF1α-dependent mediator of downregulating mitochondrial oxygen consumption.
Liu, L. et al. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol. Cell 21, 521–531 (2006).
Brugarolas, J. et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 18, 2893–2904 (2004).
DeYoung, M. P., Horak, P., Sofer, A., Sgroi, D. & Ellisen, L. W. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 22, 239–251 (2008).
Scortegagna, M. et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nature Genet. 35, 331–340 (2003).
Carey, H. V., Andrews, M. T. & Martin, S. L. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 83, 1153–1181 (2003).
Andrews, M. T. Genes controlling the metabolic switch in hibernating mammals. Biochem. Soc. Trans. 32, 1021–1024 (2004).
Andrews, M. T., Squire, T. L., Bowen, C. M. & Rollins, M. B. Low-temperature carbon utilization is regulated by novel gene activity in the heart of a hibernating mammal. Proc. Natl Acad. Sci. USA 95, 8392–8397 (1998).
Buck, M. J., Squire, T. L. & Andrews, M. T. Coordinate expression of the PDK4 gene: a means of regulating fuel selection in a hibernating mammal. Physiol. Genomics 8, 5–13 (2002).
Galster, W. A. & Morrison, P. Seasonal changes in serum lipids and proteins in the 13-lined ground squirrel. Comp. Biochem. Physiol. 18, 489–501 (1966).
Harlow, H. J. & Frank, C. L. The role of dietary fatty acids in the evolution of spontaneous and facultative hibernation patterns in prairie dogs. J. Comp. Physiol. [B] 171, 77–84 (2001).
Drew, K. L. et al. Hypoxia tolerance in mammalian heterotherms. J. Exp. Biol. 207, 3155–3162 (2004).
Zhou, F. et al. Hibernation, a model of neuroprotection. Am. J. Pathol. 158, 2145–2151 (2001).
Bernhardt, W. M. et al. Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J. Am. Soc. Nephrol. 17, 1970–1978 (2006).
Hill, P. et al. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia–reperfusion injury. J. Am. Soc. Nephrol. 19, 39–46 (2008). References 59 and 60 demonstrate that inhibition of PHDs confer renal protection against ischaemic insults.
Zhong, Z. et al. Activation of the oxygen-sensing signal cascade prevents mitochondrial injury after mouse liver ischemia–reperfusion. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G823–G832 (2008).
Taylor, C. T. & Pouyssegur, J. Oxygen, hypoxia, and stress. Ann. NY Acad. Sci. 1113, 87–94 (2007).
Kulkarni, A. C., Kuppusamy, P. & Parinandi, N. Oxygen, the lead actor in the pathophysiologic drama: enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxid. Redox Signal. 9, 1717–1730 (2007).
Bonnet, S. et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 11, 37–51 (2007).
Heusch, G., Schulz, R. & Rahimtoola, S. H. Myocardial hibernation: a delicate balance. Am. J. Physiol. Heart Circ. Physiol. 288, H984–H999 (2005).
Fallavollita, J. A., Malm, B. J. & Canty, J. M. Jr. Hibernating myocardium retains metabolic and contractile reserve despite regional reductions in flow, function, and oxygen consumption at rest. Circ. Res. 92, 48–55 (2003).
Page, B. et al. Persistent regional downregulation in mitochondrial enzymes and upregulation of stress proteins in swine with chronic hibernating myocardium. Circ. Res. 102, 103–112 (2008).
Depre, C. et al. Program of cell survival underlying human and experimental hibernating myocardium. Circ. Res. 95, 433–440 (2004).
Kim, S. J. et al. Persistent stunning induces myocardial hibernation and protection: flow/function and metabolic mechanisms. Circ. Res. 92, 1233–1239 (2003).
Hosokawa, R. et al. Myocardial metabolism of 123I-BMIPP during low-flow ischaemia in an experimental model: comparison with myocardial blood flow and 18F-FDG. Eur. J. Nucl. Med. 28, 1630–1639 (2001).
Shimonagata, T. et al. Metabolic changes in hibernating myocardium after percutaneous transluminal coronary angioplasty and the relation between recovery in left ventricular function and free fatty acid metabolism. Am. J. Cardiol. 82, 559–563 (1998).
Heusch, G. Hibernating myocardium. Physiol. Rev. 78, 1055–1085 (1998).
Sridharan, V. et al. The prolyl hydroxylase oxygen-sensing pathway is cytoprotective and allows maintenance of mitochondrial membrane potential during metabolic inhibition. Am. J. Physiol. Cell Physiol. 292, C719–C728 (2007). This publication describes mechanistic and metabolic changes on broad PHD inhibition on cardiomyocytes, and introduces the concept of alternative mechanisms to prevent cellular damage upon PHD inhibition.
Ockaili, R. et al. HIF-1 activation attenuates postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am. J. Physiol. Heart Circ. Physiol. 289, H542–H548 (2005).
Wright, G., Higgin, J. J., Raines, R. T., Steenbergen, C. & Murphy, E. Activation of the prolyl hydroxylase oxygen-sensor results in induction of GLUT1, heme oxygenase-1, and nitric-oxide synthase proteins and confers protection from metabolic inhibition to cardiomyocytes. J. Biol. Chem. 278, 20235–20239 (2003).
May, D. et al. Transgenic system for conditional induction and rescue of chronic myocardial hibernation provides insights into genomic programs of hibernation. Proc. Natl Acad. Sci. USA 105, 282–287 (2008). This is the first paper to report an animal model of transient myocardial hibernation using transgenic modulation of cardiac VEGF levels that leads to attenuation of myocardium oxygen supply. This allows the study of mechanistic principles of myocardial hibernation utilizing the power of mouse genetics.
Nakai, A. et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nature Med. 13, 619–624 (2007).
Grone, H. J., Warnecke, E. & Olbricht, C. J. Characteristics of renal tubular atrophy in experimental renovascular hypertension: a model of kidney hibernation. Nephron 72, 243–252 (1996).
Warncke, J. et al. A hibernating kidney — ischemic preconditioning in a renal transplant recipient with a proximal stenosis of the iliac artery. Clin. Nephrol. 70, 168–171 (2008).
Baranova, O. et al. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J. Neurosci. 27, 6320–6332 (2007).
Stenzel-Poore, M. P. et al. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet 362, 1028–1037 (2003). Using a genomic approach, this work illustrates similarities in neuroprotection-conferring programmes between hibernation and a transiently induced hypoxia-tolerant state.
Matsumoto, M. et al. Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J. Am. Soc. Nephrol. 14, 1825–1832 (2003).
Witteles, R. M. & Fowler, M. B. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J. Am. Coll. Cardiol. 51, 93–102 (2008).
van Herpen, N. A. & Schrauwen-Hinderling, V. B. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol. Behav. 94, 231–241 (2008).
Patel, A. et al. Early stress protein gene expression in a human model of ischemic preconditioning. Transplantation 78, 1479–1487 (2004).
Alchera, E. et al. Adenosine-dependent activation of hypoxia-inducible factor-1 induces late preconditioning in liver cells. Hepatology 48, 230–239 (2008).
Amador, A. et al. Ischemic pre-conditioning in deceased donor liver transplantation: a prospective randomized clinical trial. Am. J. Transplant. 7, 2180–2189 (2007).
Grenz, A. et al. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J. Am. Soc. Nephrol. 18, 833–845 (2007).
Gidday, J. M. Cerebral preconditioning and ischaemic tolerance. Nature Rev. Neurosci. 7, 437–448 (2006).
Sharp, F. R. et al. Hypoxic preconditioning protects against ischemic brain injury. NeuroRx 1, 26–35 (2004).
Jones, N. M. & Bergeron, M. Hypoxic preconditioning induces changes in HIF-1 target genes in neonatal rat brain. J. Cereb. Blood Flow Metab. 21, 1105–1114 (2001).
Cai, Z. et al. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1α. Cardiovasc. Res. 77, 463–470 (2008). This study identifies HIF1α as an essential component in mediating preconditioning-induced cardioprotection against ischaemia–reperfusion injury and highlights the importance of activating the prosurvival serine/threonine kinase AKT in mediating protection.
Dendorfer, A. et al. Deferoxamine induces prolonged cardiac preconditioning via accumulation of oxygen radicals. Free Radic. Biol. Med. 38, 117–124 (2005).
Philipp, S. et al. Desferoxamine and ethyl-3,4-dihydroxybenzoate protect myocardium by activating NOS and generating mitochondrial ROS. Am. J. Physiol. Heart Circ. Physiol. 290, H450–H457 (2006).
Bergeron, M. et al. Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann. Neurol. 48, 285–296 (2000). This paper identifies that HIF1α activation contributes to protective brain preconditioning.
Sarco, D. P., Becker, J., Palmer, C., Sheldon, R. A. & Ferriero, D. M. The neuroprotective effect of deferoxamine in the hypoxic-ischemic immature mouse brain. Neurosci. Lett. 282, 113–116 (2000).
Rosenberger, C. et al. Activation of hypoxia inducible factors (HIF) ameliorates hypoxic distal tubular injury in the isolated perfused rat kidney. Nephrol. Dial. Transplant. 23, 3472–3478 (2008).
Otani, H. Ischemic preconditioning: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 10, 207–247 (2008).
Obrenovitch, T. P. Molecular physiology of preconditioning-induced brain tolerance to ischemia. Physiol. Rev. 88, 211–247 (2008).
Synnestvedt, K. et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest. 110, 993–1002 (2002).
Kong, T., Westerman, K. A., Faigle, M., Eltzschig, H. K. & Colgan, S. P. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 20, 2242–2250 (2006).
Grenz, A. et al. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 5, e137 (2008).
Hausenloy, D. J., Tsang, A., Mocanu, M. M. & Yellon, D. M. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am. J. Physiol. Heart Circ. Physiol. 288, H971–H976 (2005).
Murphy, E. & Steenbergen, C. Mechanisms underlying acute protection from cardiac ischemia–reperfusion injury. Physiol. Rev. 88, 581–609 (2008).
Hausenloy, D. J., Tsang, A. & Yellon, D. M. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc. Med. 15, 69–75 (2005).
Halestrap, A. P., Clarke, S. J. & Khaliulin, I. The role of mitochondria in protection of the heart by preconditioning. Biochim. Biophys. Acta 1767, 1007–1031 (2007).
Murata, M., Akao, M., O'Rourke, B. & Marban, E. Mitochondrial ATP-sensitive potassium channels attenuate matrix Ca2+ overload during simulated ischemia and reperfusion: possible mechanism of cardioprotection. Circ. Res. 89, 891–898 (2001).
Leonard, M. O. et al. Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia–reperfusion injury. FASEB J. 20, 2624–2626 (2006).
Cai, Z. & Semenza, G. L. PTEN activity is modulated during ischemia and reperfusion: involvement in the induction and decay of preconditioning. Circ. Res. 97, 1351–1359 (2005).
Mocanu, M. M. & Yellon, D. M. PTEN, the Achilles' heel of myocardial ischaemia/reperfusion injury? Br. J. Pharmacol. 150, 833–838 (2007).
Palacios-Callender, M., Quintero, M., Hollis, V. S., Springett, R. J. & Moncada, S. Endogenous NO regulates superoxide production at low oxygen concentrations by modifying the redox state of cytochrome c oxidase. Proc. Natl Acad. Sci. USA 101, 7630–7635 (2004).
Quintero, M., Brennan, P. A., Thomas, G. J. & Moncada, S. Nitric oxide is a factor in the stabilization of hypoxia-inducible factor-1α in cancer: role of free radical formation. Cancer Res. 66, 770–774 (2006).
Jefayri, M. K., Grace, P. A. & Mathie, R. T. Attenuation of reperfusion injury by renal ischaemic preconditioning: the role of nitric oxide. BJU Int. 85, 1007–1013 (2000).
Park, K. M. et al. Inducible nitric-oxide synthase is an important contributor to prolonged protective effects of ischemic preconditioning in the mouse kidney. J. Biol. Chem. 278, 27256–27266 (2003).
Guo, Y. et al. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc. Natl Acad. Sci. USA 96, 11507–11512 (1999).
Date, T. et al. Expression of constitutively stable hybrid hypoxia-inducible factor-1α protects cultured rat cardiomyocytes against simulated ischemia–reperfusion injury. Am. J. Physiol. Cell Physiol. 288, C314–C320 (2005).
Grimm, C. et al. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nature Med. 8, 718–724 (2002).
Zhu, Y., Zhang, Y., Ojwang, B. A., Brantley, M. A. Jr & Gidday, J. M. Long-term tolerance to retinal ischemia by repetitive hypoxic preconditioning: role of HIF-1α and heme oxygenase-1. Invest. Ophthalmol. Vis. Sci. 48, 1735–1743 (2007).
Bernaudin, M. et al. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J. Cereb. Blood Flow Metab. 22, 393–403 (2002).
Wick, A. et al. Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. J. Neurosci. 22, 6401–6407 (2002).
Peng, Z. et al. Up-regulated HIF-1α is involved in the hypoxic tolerance induced by hyperbaric oxygen preconditioning. Brain Res. 1212, 71–78 (2008).
Prass, K. et al. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke 34, 1981–1986 (2003).
Nishijima, K. et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am. J. Pathol. 171, 53–67 (2007).
Malhotra, S., Savitz, S. I., Ocava, L. & Rosenbaum, D. M. Ischemic preconditioning is mediated by erythropoietin through PI-3 kinase signaling in an animal model of transient ischemic attack. J. Neurosci. Res. 83, 19–27 (2006).
Schlisio, S. et al. The kinesin KIF1Bβ acts downstream from EglN3 to induce apoptosis and is a potential 1p36 tumor suppressor. Genes Dev. 22, 884–893 (2008).
Kariko, K., Weissman, D. & Welsh, F. A. Inhibition of toll-like receptor and cytokine signaling — a unifying theme in ischemic tolerance. J. Cereb. Blood Flow Metab. 24, 1288–1304 (2004).
Saadi, S., Wrenshall, L. E. & Platt, J. L. Regional manifestations and control of the immune system. FASEB J. 16, 849–856 (2002).
Schor, H., Vaday, G. G. & Lider, O. Modulation of leukocyte behavior by an inflamed extracellular matrix. Dev. Immunol. 7, 227–238 (2000).
Zinkernagel, A. S., Johnson, R. S. & Nizet, V. Hypoxia inducible factor (HIF) function in innate immunity and infection. J. Mol. Med. 85, 1339–1346 (2007).
Cramer, T. et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 (2003). This paper demonstrates a direct role for myeloid-specific HIF1α in regulating survival and function of inflammatory cells.
Kim, H. Y. et al. HIF-1α expression in response to lipopolysaccaride mediates induction of hepatic inflammatory cytokine TNFα. Exp. Cell Res. 313, 1866–1876 (2007).
Peyssonnaux, C. et al. Cutting edge: Essential role of hypoxia inducible factor-1α in development of lipopolysaccharide-induced sepsis. J. Immunol. 178, 7516–7519 (2007).
Peyssonnaux, C. et al. HIF-1α expression regulates the bactericidal capacity of phagocytes. J. Clin. Invest. 115, 1806–1815 (2005).
Walmsley, S. R. et al. Neutrophils from patients with heterozygous germline mutations in the von Hippel Lindau protein (pVHL) display delayed apoptosis and enhanced bacterial phagocytosis. Blood 108, 3176–3178 (2006).
Walmsley, S. R. et al. Hypoxia-induced neutrophil survival is mediated by HIF-1α-dependent NF-κB activity. J. Exp. Med. 201, 105–115 (2005).
Cummins, E. P. et al. Prolyl hydroxylase-1 negatively regulates IκB kinase-β, giving insight into hypoxia-induced NFκB activity. Proc. Natl Acad. Sci. USA 103, 18154–18159 (2006). This study identifies a direct correlation between PHD activity and regulation of NF-κB activity via proposed hydroxylation of IKKβ.
Rius, J. et al. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature 453, 807–811 (2008).
Nishi, K. et al. LPS induces hypoxia-inducible factor 1 activation in macrophage-differentiated cells in a reactive oxygen species-dependent manner. Antioxid. Redox Signal. 10, 983–996 (2008).
Hartmann, H. et al. Hypoxia-independent activation of HIF-1 by enterobacteriaceae and their siderophores. Gastroenterology 134, 756–767 (2008).
Taylor, C. T. & Colgan, S. P. Hypoxia and gastrointestinal disease. J. Mol. Med. 85, 1295–1300 (2007).
Karhausen, J. et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Invest. 114, 1098–1106 (2004).
Cummins, E. P. et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134, 156–165 (2008).
Robinson, A. et al. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134, 145–155 (2008). References 142 and 143 are the first to demonstrate that inhibition of PHDs is protective in a mouse model of colitis.
Greten, F. R. et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004).
Thiel, M. et al. Targeted deletion of HIF-1 α gene in T cells prevents their inhibition in hypoxic inflamed tissues and improves septic mice survival. PLoS ONE 2, e853 (2007).
Wild, S., Roglic, G., Green, A., Sicree, R. & King, H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27, 1047–1053 (2004).
Hosogai, N. et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56, 901–911 (2007).
Ye, J., Gao, Z., Yin, J. & He, Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am. J. Physiol. Endocrinol. Metab. 293, E1118–E1128 (2007).
Yun, Z., Maecker, H. L., Johnson, R. S. & Giaccia, A. J. Inhibition of PPAR γ 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev. Cell 2, 331–341 (2002).
Shimba, S., Wada, T., Hara, S. & Tezuka, M. EPAS1 promotes adipose differentiation in 3T3-L1 cells. J. Biol. Chem. 279, 40946–40953 (2004).
Wada, T., Shimba, S. & Tezuka, M. Transcriptional regulation of the hypoxia inducible factor-2α (HIF-2α) gene during adipose differentiation in 3T3-L1 cells. Biol. Pharm. Bull. 29, 49–54 (2006).
Floyd, Z. E., Kilroy, G., Wu, X. & Gimble, J. M. Effects of prolyl hydroxylase inhibitors on adipogenesis and hypoxia inducible factor 1 alpha levels under normoxic conditions. J. Cell Biochem. 101, 1545–1557 (2007).
Irwin, R., LaPres, J. J., Kinser, S. & McCabe, L. R. Prolyl-hydroxylase inhibition and HIF activation in osteoblasts promotes an adipocytic phenotype. J. Cell Biochem. 100, 762–772 (2007).
McDonough, M. A. et al. Selective inhibition of factor inhibiting hypoxia-inducible factor. J. Am. Chem. Soc. 127, 7680–7681 (2005).
Hirsila, M. et al. Effect of desferrioxamine and metals on the hydroxylases in the oxygen sensing pathway. FASEB J. 19, 1308–1310 (2005).
McDonough, M. A. et al. Cellular oxygen sensing: crystal structure of hypoxia-inducible factor prolyl hydroxylase (PHD2). Proc. Natl Acad. Sci. USA 103, 9814–9819 (2006). This seminal study describes the crystal structure of PHD2 and thereby lays the foundation for generating more specific PHD inhibitors.
Schofield, C. J. & Ratcliffe, P. J. Oxygen sensing by HIF hydroxylases. Nature Rev. Mol. Cell Biol. 5, 343–354 (2004).
Salnikow, K. et al. Depletion of intracellular ascorbate by the carcinogenic metals nickel and cobalt results in the induction of hypoxic stress. J. Biol. Chem. 279, 40337–40344 (2004).
Yoon, Y. S., Cho, H., Lee, J. H. & Yoon, G. Mitochondrial dysfunction via disruption of complex II activity during iron chelation-induced senescence-like growth arrest of Chang cells. Ann. NY Acad. Sci. 1011, 123–132 (2004).
Needleman, P., Turk, J., Jakschik, B. A., Morrison, A. R. & Lefkowith, J. B. Arachidonic acid metabolism. Annu. Rev. Biochem. 55, 69–102 (1986).
Hewitson, K. S., McNeill, L. A. & Schofield, C. J. Modulating the hypoxia-inducible factor signaling pathway: applications from cardiovascular disease to cancer. Curr. Pharm. Des. 10, 821–833 (2004).
Nangaku, M. et al. A novel class of prolyl hydroxylase inhibitors induces angiogenesis and exerts organ protection against ischemia. Arterioscler. Thromb. Vasc. Biol. 27, 2548–2554 (2007). This paper describes the generation of two novel PHD inhibitors that might have isoform-specific inhibitory properties.
Dann, C. E. 3rd, Bruick, R. K. & Deisenhofer, J. Structure of factor-inhibiting hypoxia-inducible factor 1: an asparaginyl hydroxylase involved in the hypoxic response pathway. Proc. Natl Acad. Sci. USA 99, 15351–15356 (2002).
Elkins, J. M. et al. Structure of factor-inhibiting hypoxia-inducible factor (HIF) reveals mechanism of oxidative modification of HIF-1α. J. Biol. Chem. 278, 1802–1806 (2003).
Villar, D., Vara-Vega, A., Landazuri, M. O. & Del Peso, L. Identification of a region on hypoxia-inducible-factor prolyl 4-hydroxylases that determines their specificity for the oxygen degradation domains. Biochem. J. 408, 231–240 (2007). This work identifies protein domains that are distant from the catalytic site which contribute to the substrate specificity of PHD isoforms. This information may aid the generation of isoform-specific inhibitors by targeting these domains.
Barth, S. et al. The peptidyl prolyl cis/trans isomerase FKBP38 determines hypoxia-inducible transcription factor prolyl-4-hydroxylase PHD2 protein stability. Mol. Cell. Biol. 27, 3758–3768 (2007).
Hopfer, U., Hopfer, H., Jablonski, K., Stahl, R. A. & Wolf, G. The novel WD-repeat protein Morg1 acts as a molecular scaffold for hypoxia-inducible factor prolyl hydroxylase 3 (PHD3). J. Biol. Chem. 281, 8645–8655 (2006).
Baek, J. H. et al. OS-9 interacts with hypoxia-inducible factor 1α and prolyl hydroxylases to promote oxygen-dependent degradation of HIF-1α. Mol. Cell 17, 503–512 (2005).
Ozer, A., Wu, L. C. & Bruick, R. K. The candidate tumor suppressor ING4 represses activation of the hypoxia inducible factor (HIF). Proc. Natl Acad. Sci. USA 102, 7481–7486 (2005).
Willam, C. et al. Peptide blockade of HIFα degradation modulates cellular metabolism and angiogenesis. Proc. Natl Acad. Sci. USA 99, 10423–10428 (2002).
Lee, C., Kim, S. J., Jeong, D. G., Lee, S. M. & Ryu, S. E. Structure of human FIH-1 reveals a unique active site pocket and interaction sites for HIF-1 and von Hippel-Lindau. J. Biol. Chem. 278, 7558–7563 (2003).
Bishop, T. et al. Abnormal sympathoadrenal development and systemic hypotension in PHD3−/− mice. Mol. Cell. Biol. 28, 3386–3400 (2008).
Takeda, K. et al. Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood 111, 3229–3235 (2008).
Takeda, K. et al. Placental but not heart defects are associated with elevated hypoxia-inducible factor α levels in mice lacking prolyl hydroxylase domain protein 2. Mol. Cell. Biol. 26, 8336–8346 (2006).
Percy, M. J. et al. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N. Engl. J. Med. 358, 162–168 (2008).
Percy, M. J. et al. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc. Natl Acad. Sci. USA 103, 654–659 (2006).
Ang, S. O. et al. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nature Genet. 32, 614–621 (2002).
Gordeuk, V. R. et al. Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood 103, 3924–3932 (2004).
Smith, T. G., Robbins, P. A. & Ratcliffe, P. J. The human side of hypoxia-inducible factor. Br. J. Haematol. 141, 325–334 (2008).
Semenza, G. L. & Prabhakar, N. R. HIF-1-dependent respiratory, cardiovascular, and redox responses to chronic intermittent hypoxia. Antioxid. Redox Signal. 9, 1391–1396 (2007).
Baader, E., Tschank, G., Baringhaus, K. H., Burghard, H. & Gunzler, V. Inhibition of prolyl 4-hydroxylase by oxalyl amino acid derivatives in vitro, in isolated microsomes and in embryonic chicken tissues. Biochem. J. 300, 525–530 (1994).
Ivan, M. et al. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc. Natl Acad. Sci. USA 99, 13459–13464 (2002).
Mole, D. R. et al. 2-oxoglutarate analogue inhibitors of HIF prolyl hydroxylase. Bioorg. Med. Chem. Lett. 13, 2677–2680 (2003).
Hamrick, S. E. et al. A role for hypoxia-inducible factor-1α in desferoxamine neuroprotection. Neurosci. Lett. 379, 96–100 (2005).
Majamaa, K., Gunzler, V., Hanauske-Abel, H. M., Myllyla, R. & Kivirikko, K. I. Partial identity of the 2-oxoglutarate and ascorbate binding sites of prolyl 4-hydroxylase. J. Biol. Chem. 261, 7819–7823 (1986).
Majamaa, K., Sasaki, T. & Uitto, J. Inhibition of prolyl hydroxylation during collagen biosynthesis in human skin fibroblast cultures by ethyl 3,4-dihydroxybenzoate. J. Invest. Dermatol. 89, 405–409 (1987).
Sasaki, T., Majamaa, K. & Uitto, J. Reduction of collagen production in keloid fibroblast cultures by ethyl-3,4-dihydroxybenzoate. Inhibition of prolyl hydroxylase activity as a mechanism of action. J. Biol. Chem. 262, 9397–9403 (1987).
Warnecke, C. et al. Activation of the hypoxia-inducible factor-pathway and stimulation of angiogenesis by application of prolyl hydroxylase inhibitors. FASEB J. 17, 1186–1188 (2003).
Nwogu, J. I. et al. Inhibition of collagen synthesis with prolyl 4-hydroxylase inhibitor improves left ventricular function and alters the pattern of left ventricular dilatation after myocardial infarction. Circulation 104, 2216–2221 (2001).
Linden, T. et al. The antimycotic ciclopirox olamine induces HIF-1alpha stability, VEGF expression, and angiogenesis. FASEB J. 17, 761–763 (2003).
Ju, H., Hao, J., Zhao, S. & Dixon, I. M. Antiproliferative and antifibrotic effects of mimosine on adult cardiac fibroblasts. Biochim. Biophys. Acta 1448, 51–60 (1998).
McCaffrey, T. A. et al. Specific inhibition of eIF-5A and collagen hydroxylation by a single agent. Antiproliferative and fibrosuppressive effects on smooth muscle cells from human coronary arteries. J. Clin. Invest. 95, 446–455 (1995).
Schlemminger, I. et al. Analogues of dealanylalahopcin are inhibitors of human HIF prolyl hydroxylases. Bioorg. Med. Chem. Lett. 13, 1451–1454 (2003).
Warshakoon, N. C. et al. Structure-based design, synthesis, and SAR evaluation of a new series of 8-hydroxyquinolines as HIF-1alpha prolyl hydroxylase inhibitors. Bioorg. Med. Chem. Lett. 16, 5517–5522 (2006).
Warshakoon, N. C. et al. Design and synthesis of a series of novel pyrazolopyridines as HIF-1α prolyl hydroxylase inhibitors. Bioorg. Med. Chem. Lett. 16, 5687–5690 (2006).
Acknowledgements
This work is supported, in part, by grant GOA2006/11/KULeuven from the University of Leuven, Belgium, Methusalem Program, KU Leuven; grant IUAP06/30 from the Federal Government Belgium; and grants FWO G.0265 and FWO G.0652 from the Flanders Research Foundation, Belgium. P.F. is supported by a postdoctoral fellowship from the Marie Curie Program of the European Commission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Peter Carmeliet is a named inventor on patent application WO/2007/082899, which claims subject matter that is partially based on the results described in this article. Should the aforementioned patent application or any resulting patent be licensed, this may result in royalty payment to P.C.
Glossary
- Angiogenesis
-
The growth of new blood vessels from pre-existing vessels. Angiogenesis is a normal process in growth and development, but also has a crucial role in the growth of tumours.
- Ischaemic preconditioning
-
A stressful but non-damaging stimulus that confers protection against a subsequent harmful acute ischaemic insult.
- Abnormalization
-
Describes blood vessels in tumours, which are often tortuous, leaky and non-homogeneous in size and shape.
- Multidrug resistance 1
-
Multidrug resistance 1 (MDR1; also known as P-glycoprotein), is a transmembrane energy-dependent drug-efflux pump encoded by the gene ABCB1 in humans. By reducing intracellular drug levels, P-glycoprotein can contribute to multidrug resistance to chemotherapeutics.
- Autophagy
-
The self-cannibalism of intracellular organelles.
- Cytochrome c oxidase
-
A component of the oxidative phosphorylation machinery within the cell that normally binds oxygen.
- Mitochondrial permeability transition pore
-
A protein pore that is formed in the inner membrane of mitochondria that can lead to mitochondrial swelling and cell death.
- Siderophores
-
Iron-binding proteins of bacterial origin.
- Adipogenesis
-
The development of fat precursor cells into mature white or brown fat tissue.
- Osteoblast
-
Osteoblasts are mononucleate cells that are responsible for bone formation.
- Polycythaemia
-
An abnormal increase in the number of blood cells (primarily red blood cells).
Rights and permissions
About this article
Cite this article
Fraisl, P., Aragonés, J. & Carmeliet, P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov 8, 139–152 (2009). https://doi.org/10.1038/nrd2761
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd2761
This article is cited by
-
Dynamic assembly of DNA-ceria nanocomplex in living cells generates artificial peroxisome
Nature Communications (2022)
-
4-O-Methylascochlorin inhibits the prolyl hydroxylation of hypoxia-inducible factor-1α, which is attenuated by ascorbate
The Journal of Antibiotics (2019)
-
Dynamics of Prolyl Hydroxylases Levels During Disease Progression in Experimental Colitis
Inflammation (2019)
-
Role of purines in regulation of metabolic reprogramming
Purinergic Signalling (2019)
-
MCT2 mediates concentration-dependent inhibition of glutamine metabolism by MOG
Nature Chemical Biology (2018)