Abstract
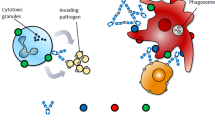
The Fc (crystallizable fragment) region of therapeutic antibodies can have an important role in their safety and efficacy. Although much is known about the structure–activity relationship of antibodies and the factors that influence Fc effector functions, a process has not yet been defined to clearly delineate how Fc functionality should be assessed and controlled during antibody development and manufacturing. In this article, we summarize the current knowledge of antibody Fc functionality, provide a strategy for assessing the effector functions of different classes of therapeutic antibodies (including Fc fusion proteins) and propose a path for routine testing and controls for manufacturers of antibody products.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lee, J. I., Zhang, L., Men, A. Y., Kenna, L. S. & Huang, S. M. CYP-mediated therapeutic protein–drug interactions — clinical findings, proposed mechanisms and regulatory implications. Clin. Pharmacokinet. 49, 295–310 (2010).
Reichert, J. M. Antibodies to watch in 2010. MAbs 2, 84–100 (2010).
Chan, A. C. & Carter, P. J. Therapeutic antibodies for autoimmunity and inflammation. Nature Rev. Immunol. 10, 301–316 (2010).
Raju, T. S. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr. Opin. Immunol. 20, 471–478 (2008).
Siberil, S. et al. FcγR: the key to optimize therapeutic antibodies? Crit. Rev. Oncol. Hematol. 62, 26–33 (2007).
Nimmerjahn, F. & Ravetch, J. Fcγ receptors as regulators of immune responses. Nature Rev. Immunol. 8, 34–47 (2008).
Ferrara, C. et al. The carbohydrate at FcγRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J. Biol. Chem. 281, 5032–5036 (2006).
Wernersson, S. et al. IgG-mediated enhancement of antibody responses is low in Fc receptor γ chain-deficient mice and increased in FcγRII-deficient mice. J. Immunol. 163, 618–622 (1999).
Hazenbos, W. L. et al. Impaired IgG-dependent anaphylaxis and arthus reaction in Fcγ RIII (CD16) deficient mice. Immunity 5, 181–188 (1996).
Lazar, G. A. et al. Engineered antibody Fc variants with enhanced effector function. Proc. Natl Acad. Sci. USA 103, 4005–4010 (2006).
Cartron, G., Watier, H., Golay, J. & Solal-Celigny, P. From the bench to the bedside: ways to improve rituximab efficacy. Blood 104, 2635–2642 (2004).
Weng, W. K. & Levy, R. Two immunoglobulin G Fc receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 21, 3940–3947 (2003).
Gennari, R. et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin. Cancer Res. 10, 5650–5655 (2004).
Musolino, A. et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J. Clin. Oncol. 26, 1789–1796 (2008).
Idusogie, E. E. et al. Engineered antibodies with increased activity to recruit complement. J. Immunol. 166, 2571–2575 (2001).
Dall'Acqua, W. F., Cook, K. E., Damschroder, M. M., Woods, R. M. & Wu, H. Modulation of the effector functions of a human IgG1 through engineering of its hinge region. J. Immunol. 177, 1129–1138 (2006).
Roopenian, D. C. & Akilesh, S. FcRn: the neonatal Fc receptor comes of age. Nature Rev. Immunol. 7, 715–725 (2007).
Jefferis, R. Antibody therapeutics: isotype and glycoform selection. Expert Opin. Biol. Ther. 7, 1401–1413 (2007).
Jefferis, R. Glycosylation as a strategy to improve antibody-based therapeutics. Nature Rev. Drug Discov. 8, 226–234 (2009).
Sondermann, P., Huber, R., Oosthuizen, V. & Jacob, U. The 3.2-Å crystal structure of the human IgG1 Fc fragment–FcγRIII complex. Nature 406, 267–273 (2000).
Durocher, Y. & Butler, M. Expression systems for therapeutic glycoprotein production. Curr. Opin. Biotechnol. 20, 700–707 (2009).
Jefferis, R. Glycosylation of recombinant antibody therapeutics. Biotechnol. Prog. 21, 11–16 (2005).
Shields, R. L. et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277, 26733–26740 (2002).
Shinkawa, T. et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 278, 3466–3473 (2003).
Niwa, R. et al. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J. Immunol. Methods 306, 151–160 (2005).
Ferrara, C. et al. Modulation of therapeutic antibody effector functions by glycosylation engineering: influence of Golgi enzyme localization domain and co-expression of heterologous β1 4-N-acetylglucosaminyltransferase III and Golgi α-mannosidase II. Biotechnol. Bioeng. 93, 851–861 (2006).
Peipp, M. et al. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood 112, 2390–2399 (2008).
Hodoniczky, J., Zheng, Y. Z. & James, D. C. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol. Prog. 21, 1644–1652 (2005).
Houde, D., Peng, Y., Berkowitz, S. A. & Engen, J. R. Post-translational modifications differentially affect IgG1 conformation and receptor binding. Mol. Cell. Proteomics 9, 1716–1728 (2010).
Anthony, R. M. et al. Recaptulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 320, 373–376 (2008).
Scallon, B., Tam, S. H., McCarthy, S. G., Cai, A. N. & Raju, T. S. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol. Immunol. 44, 1524–1534 (2007).
Nimmerjahn, F., Anthony, R. M. & Ravetch, J. V. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc. Natl Acad. Sci. USA 104, 8433–8437 (2007).
Liu, X. Y., Pop, L. M. & Vitetta, V. S. Engineering therapeutic monoclonal antibodies. Immunol. Rev. 222, 9–27 (2008).
Presta, L. G. Molecular engineering and design of therapeutic antibodies. Curr. Opin. Immunol. 20, 460–470 (2008).
Umana, P. et al. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody dependent cellular cytotoxic activity. Nature Biotech. 17, 176–180 (1999).
Ghaderi, D. et al. Implications of the presence of N-glycolylneuraminic acid in recombinant therapeutic glycoproteins. Nature Biotech. 28, 863–867 (2010).
Chung, C. H. et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-α-1,3-galactose. N. Engl. J. Med. 358, 1109–1117 (2008).
Chen, X., Liu, Y. D. & Flynn, G. C. The effect of Fc glycan forms on human IgG2 antibody clearance in humans. Glycobiology 19, 240–249 (2009).
Kanda, Y. et al. Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccarides: the high-mannose, hybrid and complex types. Glycobiology 17, 104–118 (2007).
Jones, A. J. et al. Selective clearance of glycoforms of a complex glycoprotein pharmaceutical caused by terminal N-acetylglucosamine is similar in humans and cynomolgus monkeys. Glycobiology 17, 529–540 (2007).
Salfeld, J. G. Isotype selection in antibody engineering. Nature Biotech. 25, 1369–1372 (2007).
Schrama, D., Reisfeld, R. A. & Becker, J. C. Antibody targeted drugs as cancer therapeutics. Nature Rev. Drug Discov. 5, 147–159 (2006).
Schenerman, M. A., Axley, M. J., Oliver, C. N., Ram, K. & Wasserman, G. F. in Quality by Design for Biopharmaceuticals: Principles and Case Studies (eds Rathore, A. S. & Mhatre, R.) 53–84 (Wiley Series in Biotechnology and Bioengineering, Hoboken, New Jersey, 2009).
Tai, Y. T. et al. Human anti-CD40 antagonist antibody triggers significant antitumor activity against human multiple myeloma. Cancer Res. 65, 5898–5906 (2005).
Tai, Y. T. et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood 112, 1329–1337 (2007).
Gómez-Román, V. R. et al. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J. Immunol. Methods 308, 53–67 (2006).
Bracher, M. et al. Three-colour flow cytometric method to measure antibody-dependent tumour cell killing by cytotoxicity and phagocytosis. J. Immunol. Methods 323, 160–171 (2007).
Kolber, M. A. et al. Measurement of cytotoxicity by target cell release and retention of the fluorescent dye bis-carboxyethyl-carboxyfluorescein (BCECF). J. Immunol. Methods 108, 255–264 (1988).
Maley, D. T. & Simon, P. Cytotoxicity assays using cryopreserved target cells pre-labeled with the fluorescent marker europium. J. Immunol. Methods 134, 61–70 (1990).
Wang, Y., Fei, D., Vanderlaan, M. & Song, A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis 7, 335–345 (2004).
Wallace, P. K. et al. Bispecific antibody-targeted phagocytosis of HER-2/neu expressing tumor cells by myeloid cells activated in vivo. J. Immunol. Methods 248, 167–182 (2001).
Zhao, X. et al. Targeting C-type lectin-like molecule-1 for antibody-mediated immunotherapy in acute myeloid leukemia. Haematologica 95, 71–78 (2009).
Konishi, E., Kitai, Y. & Kondo, T. Utilization of complement-dependent cytotoxicity to measure low levels of antibodies: application to nonstructural protein 1 in a model of Japanese encephalitis virus. Clin. Vaccine Immunol. 15, 88–94 (2008).
Prang, N. et al. Cellular and complement-dependent cytotoxicity of Ep-CAM-specific monoclonal antibody MT201 against breast cancer cell lines. Br. J. Cancer 92, 342–349 (2005).
Pathan, N. I. et al. Mediation of apoptosis by and antitumor activity of lumiliximab in chronic lymphocytic leukemia cells and CD23+ lymphoma cell lines. Blood 111, 1594–1602 (2008).
Blanquet-Grossard, F., Thielens, N. M., Vendrely, C., Jamin, M. & Arlaud, G. J. Complement protein C1q recognizes a conformationally modified form of the prion protein. Biochemistry 44, 4349–4356 (2005).
Cartron, G. et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγRIIIa gene. Blood 99, 754–758 (2002).
Anolik, J. H. et al. The relationship of FcγRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum. 48, 455–459 (2003).
Kim, D. H. et al. FCGR3A gene polymorphisms may correlate with response to frontline R-CHOP therapy for diffuse large B-cell lymphoma. Blood 108, 2720–2725 (2006).
Dall'Ozzo, S. et al. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration–effect relationship. Cancer Res. 64, 4664–4669 (2004).
Beck, A. et al. Trends in glycosylation, glycoanalysis and glycoengineering of therapeutic antibodies and Fc-fusion proteins. Curr. Pharm. Biotechnol. 9, 482–501 (2008).
Gennaro, L. A. & Salas-Solano, O. On-line CE-LIF-MS technology for the direct characterization of N-linked glycans from therapeutic antibodies. Anal. Chem. 80, 3838–3845 (2008).
Arora, T. et al. Differences in binding and effector functions between classes of TNF antagonists. Cytokine 45, 124–131 (2009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Xu-Rong Jiang is employed by MedImmune, a wholly-owned subsidiary of AstraZeneca, and owns AstraZeneca stocks.
An Song is employed by Genentech, a member of the Roche Group and owns Roche stock.
Svetlana Bergelson is employed by Biogen Idec and owns Biogen Idec stock.
Thomas Arroll is employed by Amgen and owns Amgen stock.
Bhavin Parekh is employed by Eli Lilly and Company and owns Eli Lilly stock.
Kimberly May is employed by Merck & Co. and owns Merck stock.
Shan Chung is employed by Genentech, a member of the Roche Group, and owns Roche stock.
Robert Strouse is an employee of MedImmune, a wholly-owned subsidiary of AstraZeneca. He owns stocks in AstraZeneca.
Anthony Mire-Sluis is employed by Amgen and owns Amgen stock.
Mark Schenerman is employed by MedImmune, a wholly-owned subsidiary of AstraZeneca, and owns AstraZeneca stocks.
Related links
Rights and permissions
About this article
Cite this article
Jiang, XR., Song, A., Bergelson, S. et al. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat Rev Drug Discov 10, 101–111 (2011). https://doi.org/10.1038/nrd3365
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3365
This article is cited by
-
Cationic-nanogel nasal vaccine containing the ectodomain of RSV-small hydrophobic protein induces protective immunity in rodents
npj Vaccines (2023)
-
Emerging phagocytosis checkpoints in cancer immunotherapy
Signal Transduction and Targeted Therapy (2023)
-
Soluble ligands as drug targets
Nature Reviews Drug Discovery (2020)
-
Characterization of IgG1 Fc Deamidation at Asparagine 325 and Its Impact on Antibody-dependent Cell-mediated Cytotoxicity and FcγRIIIa Binding
Scientific Reports (2020)