Key Points
-
Physiological cell death, or apoptosis, has an important role in several normal processes, ranging from fetal development to ageing, and defects in the physiological pathways for apoptosis have a role in many diseases. Too little or too much cell death contributes to about half of the main medical illnesses for which adequate therapy or prevention is lacking. Consequently, great interest has emerged in devising therapeutic strategies for modulating the key molecules that make life-or-death decisions in cells.
-
Apoptosis is caused by proteases known as 'caspases' — cysteine aspartyl-specific proteases. Among all the apoptosis-based drug targets, strategies that target caspases are at the forefront for blocking apoptosis in numerous diseases. Proof-of-concept data have been obtained in animal models, using peptidyl inhibitors of caspases that have shown substantial protection in rodent models of stroke.
-
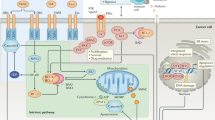
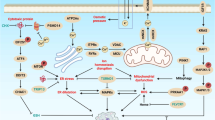
Several caspase activation pathways are known, including: the formation of a death-induced signalling complex (DISC) that contains members of the tumour-necrosis factor (TNF) family of cytokine receptors; the release of cytochrome c from mitochondria; and the injection of apoptosis-inducing proteases, such as granzyme B, into immune cells through perforin channels.
-
Regulatory molecules of caspases, such as apoptosis-activating factor 1 (APAF1), have nucleotide-binding domains. APAF1 also contains a caspase-associated recruitment domain (CARD), which binds specifically to a complementary CARD within the prodomain of procaspase-9. To the extent that selective inhibitors of caspase-9 might be difficult to generate, drugs that attack the nucleotide-binding domain of APAF1 might represent a viable alternative.
-
Inhibitor-of-apoptosis proteins (IAPs) keep caspases in check. Some IAPs are overexpressed in cancers, and are associated with resistance to apoptosis. Among these are survivin and melanoma IAP (MLIAP), which are expressed at low levels in normal adult tissues, but are found at high levels in certain types of tumour. Interest has arisen in strategies for interfering with IAP function, so that caspases can be freed to induce apoptosis of cancer cells. Antisense experiments have also helped to validate certain IAPs as potential drug targets for cancer.
-
BCL2-family proteins regulate the release of cytochrome c and other proteins from mitochondria, with pro-apoptotic BCL2-family proteins promoting, and anti-apoptotic family members suppressing, protein release by affecting the permeability of mitochondrial membranes. Several approaches have been proposed for exploiting BCL2-family proteins for therapeutic gain. Strategies for generating small-molecule inhibitors of members of the BCL2 family, based on functional and structural studies of their dimerization, have been investigated. Compounds have been reported that bind to anti-apoptotic BCL2 or BCL-XL and promote apoptosis of cancer cells.
-
Opportunities exist to indirectly affect apoptosis by modulating inputs into cell-death pathways through protein kinases, protein phosphatases, transcription factors and cell-surface receptors for cytokines, neurotrophins, cardiotrophins and growth factors. Although many signal-transducing proteins ultimately link to apoptosis pathways at some level, there are a few candidate drug discovery targets that directly modulate the expression or function of core death-machinery genes and proteins.
-
Advances in understanding the molecular mechanisms of apoptosis proteins have revealed strategies for potential therapeutic intervention in a wide range of ailments in which cell survival and death are unbalanced. Some of these strategies have progressed to clinical testing in humans, and will undoubtedly teach us much about the prospects for modulating apoptosis as a therapeutic approach.
Abstract
Many of today's medical illnesses can be attributed directly or indirectly to problems with apoptosis — a programmed cell-suicide mechanism. Disorders in which defective regulation of apoptosis contributes to disease pathogenesis or progression can involve either cell accumulation, in which cell eradication or cell turnover is impaired, or cell loss, in which the cell-suicide programme is inappropriately triggered. Identification of the genes and gene products that are responsible for apoptosis, together with emerging information about the mechanisms of action and structures of apoptotic regulatory and effector proteins, has laid a foundation for the discovery of drugs, some of which are now undergoing evaluation in human clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Engelberg-Kulka, H. & Glaser, G. Addiction modules and programmed cell death and antideath in bacterial cultures. Annu. Rev. Microbiol. 53, 43–70 (1999).
Thompson, C. B. Apoptosis in the pathogenesis and treatment of disease. Science 267, 1456–1462 (1995).
Reed, J. C. Mechanisms of apoptosis. Am. J. Pathol. 157, 1415–1430 (2000).
Thornberry, N. & Lazebnik, Y. Caspases: enemies within. Science 281, 1312–1316 (1998).
Li, P. et al. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 80, 401–411 (1995).
Bergeron, L. et al. Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev. 12, 1304–1314 (1998).
Wang, S. et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92, 501–509 (1998).
Ona, V. O. et al. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature 399, 263–267 (1999).This paper showed that caspase inhibition could slow the progression of a chronic neurodegenerative disease in an animal model, and therefore strengthened the cause-and-effect linkage between caspases and severity or kinetics of neurodegeneration.
Endres, M. et al. Attentuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J. Cereb. Blood Flow Metab. 18, 238–247 (1998).
Holly, T. A. et al. Caspase inhibition reduces myocyte cell death induced by myocardial ischemia and reperfusion in vivo. J Mol Cell Cardiol 31, 1709–1715 (1999).
Wiessner, C., Sauer, D., Alaimo, D. & Allegrini, P. R. Protective effect of a caspase inhibitor in models for cerebral ischemia in vitro and in vivo. Cell. Mol. Biol. 46, 53–62 (2000). | PubMed |
Rabuffetti, M. et al. Inhibition of caspase-1-like activity by Ac-Tyr-Val-Ala-Asp-chloromethyl ketone induces long-lasting neuroprotection in cerebral ischemia through apoptosis reduction and decrease of proinflammatory cytokines. J. Neurosci. 20, 4398–4404 (2000).
Li, M. et al. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science 288, 335–339 (2000).
Lee, D. et al. Potent and selective nonpeptide inhibitors of caspases 3 and 7. J. Med. Chem. 44, 2015–2026 (2001).
Nicholson, D. W. From bench to clinic with apoptosis-based therapeutic agents. Nature 407, 810–816 (2000).
Reed, J. C. & Tomaselli, K. Drug discovery opportunities from apoptosis research. Curr. Opin. Biotechnol. 11, 586–592 (2000).
Green, D. R. & Reed, J. C. Mitochondria and apoptosis. Science 281, 1309–1312 (1998).
Li, P. et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479–489 (1997).The first paper to describe the 'apoptosome' —a holoenzyme complex that maintains caspase-9 in an active conformation — and define its minimal components: APAF1, caspase-9 and cytochrome c.
Qin, H. et al. Structural basis of procaspase-9 recruitment by the apoptotic protease-activating factor 1. Nature 399, 549–557 (1999).
Zou, H., Li, Y., Liu, X. & Wang, X. An APAF-1 cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 274, 11549–11556 (1999).
Saleh, A., Srinivasula, S., Acharya, S., Fishel, R. & Alnemri, E. Cytochrome c and dATP-mediated oligomerization of Apaf-1 is a prerequisite for procaspase-9 activation. J. Biol. Chem. 274, 17941–17945 (1999).
Salvesen, G. S. & Dixit, V. M. Caspase activation: the induced-proximity model. Proc. Natl Acad. Sci. USA 96, 10964–10967 (1999).
Hakem, R. et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell 94, 339–352 (1998).
Chou, J., Matsuo, H., Duan, H. & Wagner, G. Solution structure of the RAIDD CARD and model for CARD/CARD interaction in caspase-2 and caspase-9 recruitment. Cell 94, 171–180 (1998).
Ruland, J. et al. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-κB and neural tube closure. Cell 104, 33–42 (2001).
Yeh, W. et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 279, 1954–1958 (1998).
Zhang, J., Cado, C., Chen, A., Kabra, N. & Winoto, A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort 1. Nature 392, 296–299 (1998).
Kuang, A. A., Diehl, G. E., Zhang, J. & Winoto, A. FADD is required for DR4- and DR5-mediated apoptosis: lack of TRAIL-induced apoptosis in FADD-deficient mouse embryonic fibroblasts. J. Biol. Chem. 275, 25065–25068 (2000).
Deveraux, Q. & Reed, J. IAP family proteins: suppressors of apoptosis. Genes Dev. 13, 239–252 (1999).
La Casse, E. C., Baird, S., Korneluk, R. G. & MacKenzie, A. E. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene 17, 3247–3259 (1998).
Fesik, S. W. Insights into programmed cell death through structural biology. Cell 103, 273–282 (2000).
Yang, Y., Fang, S., Jensen, J., Weissman, A. & Ashwell, J. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science 288, 874–877 (2000).
Ambrosini, G., Adida, C. & Altieri, D. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nature Med. 3, 917–921 (1997).The first report of the IAP-family member survivin, and the first study to show that some IAPs are over-produced in cancers.
Vucic, D., Stennicke, H. R., Pisabarro, M. T., Salvesen, G. S. & Dixit, V. M. ML-IAP, a novel inhibitor of apoptosis that is preferentially expressed in human melanomas. Curr. Biol. 10, 1359–1366 (2000).
Chen, J. et al. Down-regulation of survivin by antisense oligonucleotides increases apoptosis, inhibits cytokinesis and anchorage-independent growth. Neoplasia 2, 235–241 (2000).
Holcik, M. et al. The hippocampal neurons of neuronal apoptosis inhibitory protein 1 (NAIP1)-deleted mice display increased vulnerability to kainic acid-induced injury. Proc. Natl Acad. Sci. USA 97, 2286–2290 (2000).
Xu, D. G. et al. Elevation of neuronal expression of NAIP reduces ischemic damage in the rat hippocampus. Nature Med. 3, 997–1004 (1997).An early study that shows that gene-transfer-mediated elevations in the expression of IAP-family proteins can provide neuroprotection in vivo . This paper applied NAIP (the first human IAP to be discovered) in an animal model of cerebral ischaemia.
Xu, D. et al. Attenuation of ischemia-induced cellular and behavioral deficits by X chromosome-linked inhibitor of apoptosis protein overexpression in the rat hippocampus. J. Neurosci. 19, 5026–5033 (1999).
Adams, J. & Cory, S. The Bcl-2 protein family: arbiters of cell survival. Science 281, 1322–1326 (1998).
Gross, A., McDonnell, J. & Korsmeyer, S. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13, 1899–1911 (1999).
Huang, D. C. & Strasser, A. BH3-only proteins — essential initiators of apoptotic cell death. Cell 103, 839–842 (2000).
Dubois-Dauphin, M., Frankowski, H., Tsujimoto, Y., Huarte, J. & Martinou, J.-C. Neonatal motoneurons overexpressing the Bcl-2 protooncogene in transgenic mice are protected from axotomy-induced cell death. Proc. Natl Acad. Sci. USA 91, 3309–3313 (1994).
Cenni, M. C. et al. Long-term survival of retinal ganglion cells following optic nerve section in adult Bcl-2 transgenic mice. Eur. J. Neurosci. 8, 1735–1745 (1996).
Linnik, M. D., Zahos, P., Geschwind, M. D. & Federoff, H. J. Expression of Bcl-2 from a defective herpes simplex virus-1 vector limits neuronal death in focal cerebral ischemia. Stroke 26, 1670–1674 (1995).
Kostic, V., Jackson-Lewis, V., de Bilbao, F., Dubois-Dauphin, M. & Przedborski, S. Bcl-2: prolonging life in a transgenic mouse model of familial amyotrophic lateral sclerosis. Science 277, 559–562 (1997).This report provided direct evidence that an apoptosis-blocking protein (BCL2) could prolong survival in an animal model of a chronic neurodegenerative disease, and therefore strengthened the cause-and-effect linkage between the apoptotic machinery and severity or kinetics of neurodegeneration.
Haldar, S., Jena, N. & Croce, C. M. Inactivation of Bcl-2 by phosphorylation. Proc. Natl Acad. Sci. USA 92, 4507–4511 (1995).
Cheng, E. et al. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science 278, 1966–1968 (1997).
Sagot, S. et al. Polymer encapsulated cell lines genetically engineered to release ciliary neurotrophic factor can slow down progressive motor neuronopathy in the mouse. Eur. J. Neurosci. 7, 1313–1322 (1995).
Sattler, M. et al. Structure of Bcl-XL–Bak peptide complex: recognition between regulators of apoptosis. Science 275, 983–986 (1997).The three-dimensional structure of BCL-X L bound to an inhibitory BH3 peptide laid the foundation for subsequent attempts to screen for, or derive by rational means, small-molecule antagonists of BCL2-family proteins.
Wang, J.-L. et al. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc. Natl Acad. Sci. USA 97, 7124–7129 (2000).This paper provided the first evidence that small-molecule inhibitors of BCL2 could be produced.
Degterev, A. et al. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-XL . Nature Cell Biol. 3, 173–182 (2001).
Tzung, S. et al. Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nature Cell Biol. 3, 183–192 (2001).
Kroemer, G. & Reed, J. C. Mitochondrial control of cell death. Nature Med. 6, 513–519 (2000).
Bernardi, P. et al. The mitochondrial permeability transition. Biofactors 8, 273–281 (1998).
Sagot, Y. et al. An orally active anti-apoptotic molecule (CGP3466B) preserves mitochondria and enhances survival in an animal model of motoneuron disease. Br. J. Pharmacol. 131, 721–728 (2000).
Kragten, E. et al. Glyceraldehyde-3-phosphate dehydrogenase, the putative target of the anti-apoptotic compounds CGP3466 and R-(−)-deprenyl. J. Biol. Chem. 273, 5821–5826 (1998).
Tatton, W. G. & Chalmers-Redman, R. M. E. Modulation of gene expression rather than monoamine oxidase inhibition (−)-deprenyl-related compounds in controlling neurodegeneration. Neurology 47, S171–S183 (1996).
Yang, D. D. et al. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature 389, 865–870 (1997).This work used a Jnk3 knockout mouse to validate this protein kinase as a potential drug discovery target for neuronal cell death.
Ichijo, H. et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275, 90–94 (1997).
Geleziunas, R., Xu, W., Takeda, K., Ishijo, H. & Greene, W. C. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature 410, 834–838 (2001).
Kissil, J. L. & Kimchi, A. Death-associated proteins: from gene identification to the analysis of their apoptotic and tumour suppressive functions. Mol. Med. Today 4, 268–274 (1998).
Datta, S., Brunet, A. & Greenberg, M. Cellular survival: a play in three Akts. Genes Dev. 13, 2905–2927 (1999).
Wang, H.-G. et al. Calcineurin promotes apoptosis by dephosphorylating BAD. Science 284, 339–343 (1998).
Kaul, M., Garden, G. A. & Lipton, S. A. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410, 988–994 (2001).
Di Cristafano, A. & Pandolfi, P. P. The multiple roles of PTEN in tumor suppression. Cell 100, 387–390 (2000).
Kim, A. H., Khursigara, G., Sun, X., Franke, T. F. & Chao, M. V. AKT1 phosphorylates and negatively regulates ASK1. Mol. Cell. Biol. 21, 893–901 (2001).
Pekarsky, Y. et al. Akt phosphorylates and regulates the orphan nuclear receptor Nur77. Proc. Natl Acad. Sci. USA 98, 3690–3694 (2001).
Cho, H. et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ). Science 292, 1728–1731 (2001).
Lietzke, S. E. et al. Structural basis of 3-phosphoinositide recognition by pleckstrin homology domains. Mol. Cell 6, 385–394 (2000).
Neshat, M. S. et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc. Natl Acad. Sci. USA 98, 10314–10319 (2001).
Silverman, N. & Maniatis, T. NF-κB signaling pathways in mammalian and insect innate immunity. Genes Dev. 15, 2321–2342 (2001).
Lee, H. H., Dadgostar, H., Cheng, Q., Shu, J. & Cheng, G. NF-κB-mediated up-regulation of BCL-x and BFL-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc. Natl Acad. Sci. USA 96, 9136–9141 (1999).
Chu, Z. L. et al. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-κB control. Proc. Natl Acad. Sci. USA 94, 10057–10062 (1997).
Stehlik, C. et al. Nuclear factor (NF)-κB-regulated X-chromosome-linked IAP gene expression protects endothelial cells from tumor necrosis factor-α-induced apoptosis. J. Exp. Med. 188, 211–216 (1998).This investigation showed a direct link between NF-κB and the expression of IAP-family genes, indicating that the induction of IAP expression represents an important mechanism by which NF-κB prevents TNFα-induced apoptosis in normal cells.
Rossi, A. et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature 403, 103–108 (2000).Shows that certain types of prostaglandins (cyclopentone-type) directly inhibit IKKs. The study provided insights into the anti-inflammatory properties of some prostaglandins, and proposed routes to the discovery of small-molecule antagonists of IKKs.
Holmes-McNary, M. & Baldwin, A. S. J. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IκB kinase. Cancer Res. 60, 3477–3483 (2000).
Sporn, M. B., Suh, N. & Mangelsdorf, D. J. Prospects for prevention and treatment of cancer with SPARMs (selective PPARγ modulators). Trends Mol. Med. 7, 395–400 (2001).
Adams, J., Palombella, V. J. & Elliott, P. J. Proteasome inhibition: a new strategy in cancer treatment. Invest. New Drugs 18, 109–121 (2000).
Thompson, W. J., et al. Exisulind induction of apoptosis involves guanosine 3′,5′-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated β-catenin. Cancer Res. 60, 3338–3342 (2000).
Goluboff, E. T. et al. Safety and efficacy of Exisulind for treatment of recurrent prostate cancer after radical prostatectomy. J. Urol. 166, 882–886 (2001).
Pratt, M. A. C. et al. Bcl-2 is required to prevent estrogen withdrawal-induced human breast cancer tumour regression. FEBS Lett. 440, 403–408 (1998).
Andreeff, M. et al. Expression of Bcl-2 family genes in normal and AML progenitors: changes induced by chemotherapy and retinoic acid. Leukemia 13, 1881–1892 (1999).
Blutt, S. E., McDonnell, T. J., Polek, T. C. & Weigel, N. L. Calcitriol-induced apoptosis in LNCaP cells is blocked by overexpression of Bcl-2. Endocrinology 141, 10–17 (2000).
Sheng, H., Shao, J., Morrow, J. D., Beauchamp, R. D. & DuBois, R. N. Modulation of apoptosis and Bcl-2 expression by progtaglandin E2 in human colon cancer cells. Cancer Res. 58, 362–366 (1998).
Tsujii, M. & DuBois, R. N. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell 83, 493–501 (1995).
Zhang, L., Yu, J., Park, B. H., Kinzler, K. W. & Vogelstein, B. Role of BAX in the apoptotic response to anticancer agents. Science 290, 989–992 (2000).
Sun, M. et al. Phosphatidylinositol-3-OH kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor-α (ERα) via interaction between ERα and PI3K. Cancer Res. 61, 5985–5991 (2001).
Kousteni, S. et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104, 719–730 (2001).A study that provided in vivo evidence that steroid-hormone receptors can have transcription-independent mechanisms linked to apoptosis regulation.
Li, H. et al. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science 289, 1159–1164 (2000).Showed that steroid/retinoid-family transcription factors can function outside the nucleus to regulate apoptosis, indicating opportunities for the development of small-molecule drugs that modulate this non-nuclear, non-transcriptional activity.
Brenner, C. & Kroemer, G. Apoptosis. Mitochondria — the death signal integrators. Science 289, 1150–1151 (2000).
Richon, V. M., Zhou, X., Rifkind, R. A. & Marks, P. A. Histone deacetylase inhibitors: development of suberoylanilide hydroxamic acid (SAHA) for the treatment of cancers. Blood Cells Mol. Dis. 27, 260–264 (2001).
Grooteclaes, M. L. & Frisch, S. M. Evidence for a function of CtBP in epithelial gene regulation and anoikis. Oncogene 19, 3823–3828 (2000).
Bischoff, J. R. et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274, 373–376 (1996).Revealed that a mutant adenovirus only replicates in p53-deficient cells, setting the stage for subsequent clinical trials that were intended to exploit this property for treating cancer patients with p53-deficient tumours.
Khuri, F. R. et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nature Med. 6, 879–885 (2000).
Miyashita, T. & Reed, J. C. Tumor suppressor p53 is a direct transcriptional activator of human BAX gene. Cell 80, 293–299 (1995).This report provided the first evidence that p53 directly binds to the promoter of a pro-apoptotic gene ( BAX ) and induces its transcription, thereby providing the first direct link between p53 and the apoptosis machinery. Subsequently, p53 was shown to directly transactivate several pro-apoptotic genes, including other members of the BCL2 family and some of the TNF-family of death receptors.
Oda, E. et al. NOXA, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288, 1053–1058 (2000).
Nakano, K. & Vousden, K. H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 7, 683–694 (2001).
Yu, J., Zhang, L., Hwang, P. M., Kinzler, K. W. & Vogelstein, B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell 7, 673–682 (2001).
Owen-Schaub, L. B. et al. Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol. Cell. Biol. 15, 3032–3040 (1995).
Wu, G. S. et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nature Genet. 17, 141–143 (1997).
Roth, J. A., Swisher, S. G. & Meyn, R. E. p53 tumor suppressor gene therapy for cancer. Oncology 13, 148–154 (1999).
Foster, B., Coffey, H., Morin, M. & Rastinejad, F. Pharmacological rescue of mutant p53 conformation and function. Science 286, 2507–2510 (1999).
Komarov, P. et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 285, 1733–1737 (1999).
Ashkenazi, A. & Dixit, V. M. Apoptosis control by death and decoy receptors. Curr. Opin. Cell Biol. 11, 255–260 (1999).
Pedersen, I. M., Buhl, A. M., Klausen, P., Geisler, C. H. & Jurlander, J. The chimeric anti-CD20 antibody Rituximab induces apoptosis in B-cell chronic lymphocytic leukemia cells through a p38 MAP-kinase dependent mechanism. Blood (in the press).
Byrd, J. C. et al. The mechanism of tumor cell clearance by Rituximab in vivo in patients with B-cell chronic lymphocytic leukemia involves apoptosis via a caspase-9 pathway. Blood (in the press).
Gutheil, J. C. et al. Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin αvβ3 . Clin. Cancer Res. 6, 3056–3061 (2000).
Digicaylioglu, M. & Lipton, S. A. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-κB signalling cascades. Nature 412, 641–647 (2001).This paper showed a neuroprotective effect for erythropoietin, indicating possibilities of a new clinical application for this growth factor.
Ray, J. & Gage, P. H. Construction of cells expressing neurotrophins. Methods Mol. Biol. 169, 115–133 (2001).
Paris, F. et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 293, 293–297 (2001).
Gottlieb, R., Giesing, H., Zhu, J., Engler, R. & Babior, B. Cell acidification in apoptosis: granulocyte colony-stimulating factor delays programmed cell death in neutrophils by up-regulating the vacuolar H+-ATPase. Proc. Natl Acad. Sci. USA 92, 5965–5968 (1995).
Sun, J. et al. A new family of 10 murine ovalbumin serpins includes two homologs of proteinase inhibitor 8 and two homologs of the granzyme B inhibitor (proteinase inhibitor 9). J. Biol. Chem. 272, 15434–15441 (1997).
Nakagawa, T. et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403, 98–103 (2000).Suggested a pathway that links stress in the endoplasmic reticulum (ER) to caspase activation.
Mancini, M. et al. Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis. J. Cell Biol. 149, 603–612 (2000).
Wang, Z.-G. et al. PML is essential for multiple apoptotic pathways. Nature Genet. 20, 266–272 (1998).
Hess, J. L. & Korsmeyer, S. J. Life, death and nuclear spots. Nature Genet. 20, 220–222 (1998).Provided in vivo evidence that the nuclear protein PML modulates the apoptosis-sensitivity of cells.
Riedl, S. J. et al. Structural basis for the inhibition of caspase-3 by XIAP. Cell 104, 791–800 (2001).
Du, C., Fang, M., Li, Y., Li, L. & Wang, X. SMAC, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102, 33–42 (2000).
Verhagen, A. M. et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102, 43–53 (2000).References 118 and 119 describe the discovery of a mitochondrial protein that is released into the cytosol during apoptosis, and which binds and inhibits IAP-family proteins. Subsequent structural studies of these endogenous IAP antagonists indicated a possible way of discovering small-molecule mimics.
Suzuki, Y. et al. A serine protease HTRA2/Omi, which is released from the mitochondria and interacts with XIAP, induces caspase-independent cell death. Mol. Cell 8, 613–621 (2001).
Srinivasula, S. M. et al. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature 410, 112–116 (2001)
Acknowledgements
I thank R. Cornell and A. Sawyer for manuscript preparation, and G. Salvesen and S. Frisch for helpful discussions.
Author information
Authors and Affiliations
Related links
Glossary
- APOPTOSIS
-
A constellation of morphological changes that is observed by microscopy in cells that are undergoing programmed cell death.
- CASPASES
-
A family of intracellular cysteine proteases that are responsible for apoptosis.
- ZYMOGEN
-
The inactive pro-form of an enzyme. Typically, zymogens are activated by proteolysis.
- CHROMATIN
-
Nuclear DNA complexed with proteins, including histones, as well as with RNA.
- INDUCED PROXIMITY
-
A mechanism of caspase activation whereby the unprocessed pro-forms of caspases (zymogens) are brought into close proximity through interactions with other proteins. Because the zymogen forms of caspases have low levels of protease activity, bringing them into close proximity allows them to cleave each other, inducing their transition to the fully active state.
- NF-κB
-
A heterodimeric transcription factor of the REL family. NF-κB is known to bind to the promoters, and induce the transcription, of several anti-apoptotic proteins.
- INHIBITOR-OF-APOPTOSIS PROTEIN
-
(IAP). IAPs contain at least one copy of a Baculovirus IAP repeat (BIR) domain and suppress apoptosis when overexpressed. Several IAPs directly bind and inhibit caspases.
- BCL2
-
The founding member of a family of apoptosis-regulating proteins. Many BCL2-family members regulate mitochondria-dependent steps in cell-death pathways, with some suppressing, and others promoting, the release of apoptogenic proteins from these organelles.
- DEATH-INDUCING SIGNALLING COMPLEX
-
(DISC). The DISC refers to a complex of proteins that is assembled around the cytosolic domains of certain tumour-necrosis factor (TNF)-family death receptors that contain the death-domain structure. Invariant proteins of the DISC include the adaptor protein FADD and caspase-8. Other proteins can be found in some circumstances, depending on the TNF-family receptor and the cell type.
- APOPTOSOME
-
A multiprotein complex that consists of several (probably seven) molecules of APAF1 bound to cytochrome c and caspase-9. The apoptosome represents a holoenzyme complex, which maintains caspase-9 in an active conformation.
- LOCOREGIONAL
-
Refers to an anatomic location or a region of the body. Typically used in the context of gene therapy, for which the delivery of viral vectors is limited to a tissue or body location.
- IATROGENIC INSULT
-
Side effects or undesirable consequences that result from physician intervention.
Rights and permissions
About this article
Cite this article
Reed, J. Apoptosis-based therapies. Nat Rev Drug Discov 1, 111–121 (2002). https://doi.org/10.1038/nrd726
Issue Date:
DOI: https://doi.org/10.1038/nrd726
This article is cited by
-
Severe cellular stress drives apoptosis through a dual control mechanism independently of p53
Cell Death Discovery (2022)
-
A Novel Anti-CD22 scFv.Bim Fusion Protein Effectively Induces Apoptosis in Malignant B cells and Promotes Cytotoxicity
Applied Biochemistry and Biotechnology (2022)
-
Marine-derived pipeline anticancer natural products: a review of their pharmacotherapeutic potential and molecular mechanisms
Future Journal of Pharmaceutical Sciences (2021)
-
Combination strategies to overcome resistance to the BCL2 inhibitor venetoclax in hematologic malignancies
Cancer Cell International (2020)
-
One-Pot Radiosynthesis and Biological Evaluation of a Caspase-3 Selective 5-[123,125I]iodo-1,2,3-triazole derived Isatin SPECT Tracer
Scientific Reports (2019)