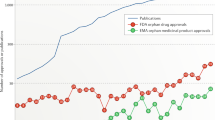
Abstract
Over the past 20 years, incentives of the Orphan Drug Act (ODA), the largest single source of extramural clinical grants at the US Food and Drug Administration, have had a substantial impact on public health. ODA incentives have contributed to the development of many innovative biotechnology products, and as our understanding of the human genome evolves, it is anticipated that pharmacogenomics will result in the identification of more 'orphan diseases'.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Title 21. United States Code (USC) Section 360ee.
Rohde, D. D. The Orphan Drug Act: an engine of innovation, at what cost? Food Drug Law J. 55, 125–143 (2000).
Title 35. United States Code (USC) Sections 102–103.
Mathieu, M. New Drug Developments: A Regulatory Overview 4th edn (PAREXEL International, Waltham, Massachusetts, 1997).
Title 21. Code of Federal Regulations (CFD) Sections 314.50 and 601.40.
Spilker, B. Guide to Clinical Trials (Raven, New York, 1991).
Haffner, M. E. Designing clinical trials to study rare disease treatment. Drug Inf. J. 32, 957–960 (1998).
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Cancer.gov
Medscape DrugInfo
OMIM
severe combined immunodeficiency
FURTHER INFORMATION
Federal Food, Drug and Cosmetic Act
Rights and permissions
About this article
Cite this article
Haffner, M., Whitley, J. & Moses, M. Two decades of orphan product development. Nat Rev Drug Discov 1, 821–825 (2002). https://doi.org/10.1038/nrd919
Issue Date:
DOI: https://doi.org/10.1038/nrd919
This article is cited by
-
Children with a rare congenital genetic disorder: a systematic review of parent experiences
Orphanet Journal of Rare Diseases (2022)
-
Disease awareness or subtle product placement? Orphan diseases featured in the television series “House, M.D.” - a cross-sectional analysis
BMC Medical Ethics (2020)
-
MicroRNAs and Long Non-coding RNAs in Genetic Diseases
Molecular Diagnosis & Therapy (2019)
-
Vom Symptom zur Diagnose – Tauglichkeit von Symptom-Checkern
HNO (2019)
-
Investigating the landscape of US orphan product approvals
Orphanet Journal of Rare Diseases (2018)