Abstract
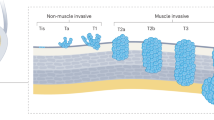
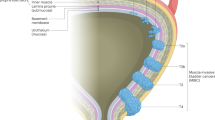
Bladder cancer is a highly prevalent disease and is associated with substantial morbidity, mortality and cost. Environmental or occupational exposures to carcinogens, especially tobacco, are the main risk factors for bladder cancer. Most bladder cancers are diagnosed after patients present with macroscopic haematuria, and cases are confirmed after transurethral resection of bladder tumour (TURBT), which also serves as the first stage of treatment. Bladder cancer develops via two distinct pathways, giving rise to non-muscle-invasive papillary tumours and non-papillary (solid) muscle-invasive tumours. The two subtypes have unique pathological features and different molecular characteristics. Indeed, The Cancer Genome Atlas project identified genetic drivers of muscle-invasive bladder cancer (MIBC) as well as subtypes of MIBC with distinct characteristics and therapeutic responses. For non-muscle-invasive bladder cancer (NMIBC), intravesical therapies (primarily Bacillus Calmette–Guérin (BCG)) with maintenance are the main treatments to prevent recurrence and progression after initial TURBT; additional therapies are needed for those who do not respond to BCG. For localized MIBC, optimizing care and reducing morbidity following cystectomy are important goals. In metastatic disease, advances in our genetic understanding of bladder cancer and in immunotherapy are being translated into new therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386 (2015).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 66, 7–30 (2016).
Mahdavifar, N., Ghoncheh, M., Pakzad, R., Momenimovahed, Z. & Salehiniya, H. Epidemiology, incidence and mortality of bladder cancer and their relationship with the development index in the world. Asian Pac. J. Cancer Prev. 17, 381–386 (2016).
Ramirez, D. et al. Microscopic haematuria at time of diagnosis is associated with lower disease stage in patients with newly diagnosed bladder cancer. BJU Int. 117, 783–786 (2016).
Elias, K., Svatek, R. S., Gupta, S., Ho, R. & Lotan, Y. High-risk patients with hematuria are not evaluated according to guideline recommendations. Cancer 116, 2954–2959 (2010).
Willis, D. & Kamat, A. M. Nonurothelial bladder cancer and rare variant histologies. Hematol. Oncol. Clin. North Am. 29, 237–252 (2015).
Smith, A. B. et al. Muscle-invasive bladder cancer: evaluating treatment and survival in the National Cancer Data Base. BJU Int. 114, 719–726 (2014).
Burger, M. et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 63, 234–241 (2013). This paper describes the incidence, prevalence and mortality of urothelial carcinoma, with a focus on factors that increase the risk of bladder cancer.
Charlton, M. E., Adamo, M. P., Sun, L. & Deorah, S. Bladder cancer collaborative stage variables and their data quality, usage, and clinical implications: a review of SEER data, 2004–2010. Cancer 120 (Suppl. 23), 3815–3825 (2014).
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507, 315–322 (2014). A milestone study that provides a comprehensive landscape of molecular alterations in bladder cancer; is also highly informative with regard to tumour biology and potential clinical interventions.
Antoni, S. et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur. Urol. 71, 96–108 (2017).
Parkin, D. M. The global burden of urinary bladder cancer. Scand. J. Urol. Nephrol. Suppl. 42, 12–20 (2008).
Yee, D. S., Ishill, N. M., Lowrance, W. T., Herr, H. W. & Elkin, E. B. Ethnic differences in bladder cancer survival. Urology 78, 544–549 (2011).
Bouchardy, C. et al. Ethnicity and cancer risk in São Paulo, Brazil. Cancer Epidemiol. Biomarkers Prev. 1, 21–27 (1991).
Ploeg, M., Aben, K. K. H. & Kiemeney, L. A. The present and future burden of urinary bladder cancer in the world. World J. Urol. 27, 289–293 (2009).
Ferlay, J. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. IARChttp://globocan.iarc.fr/Pages/burden_sel.aspx (2013).
World Health Organization. World Health Statistics 2016: monitoring health for the SDGs. WHOhttp://www.who.int/gho/publications/world_health_statistics/2016/en/ (2016).
Freedman, N. D., Silverman, D. T., Hollenbeck, A. R., Schatzkin, A. & Abnet, C. C. Association between smoking and risk of bladder cancer among men and women. JAMA 306, 737–745 (2011).
Moolgavkar, S. H. & Stevens, R. G. Smoking and cancers of bladder and pancreas: risks and temporal trends. J. Natl Cancer Inst. 67, 15–23 (1981).
Ng, M. et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA 311, 183–192 (2014).
Mackay, J. & Eriksen, M. The Tobacco Atlas. WHOhttp://www.who.int/tobacco/media/en/title.pdf (2012).
Global Burden of Disease Cancer Collaboration. The global burden of cancer 2013. JAMA Oncol. 1, 505–527 (2015).
Smith, N. D. et al. Bladder cancer mortality in the United States: a geographic and temporal analysis of socioeconomic and environmental factors. J. Urol. 195, 290–296 (2016).
Pelucchi, C. & La Vecchia, C. Alcohol, coffee, and bladder cancer risk: a review of epidemiological studies. Eur. J. Cancer Prev. 18, 62–68 (2009).
Cantiello, F. et al. Association between metabolic syndrome, obesity, diabetes mellitus and oncological outcomes of bladder cancer: a systematic review. Int. J. Urol. 22, 22–32 (2015).
Hashim, D. & Boffetta, P. Occupational and environmental exposures and cancers in developing countries. Ann. Glob. Health 80, 393–411 (2014).
Cumberbatch, M. G., Windsor-Shellard, B. & Catto, J. W. F. The contemporary landscape of occupational bladder cancer within the United Kingdom: a meta-analysis of risks over the last 80 years. BJU Int. 119, 100–109 (2017).
Purdue, M. P., Hutchings, S. J., Rushton, L. & Silverman, D. T. The proportion of cancer attributable to occupational exposures. Ann. Epidemiol. 25, 188–192 (2015).
An, Y. et al. Meta-analysis of the relationship between slow acetylation of N-acetyl transferase 2 and the risk of bladder cancer. Genet. Mol. Res. 14, 16896–16904 (2015).
Guey, L. T. et al. Genetic susceptibility to distinct bladder cancer subphenotypes. Eur. Urol. 57, 283–292 (2010).
Kiemeney, L. A. et al. A sequence variant at 4p16.3 confers susceptibility to urinary bladder cancer. Nat. Genet. 42, 415–419 (2010).
Kiemeney, L. A. et al. Sequence variant on 8q24 confers susceptibility to urinary bladder cancer. Nat. Genet. 40, 1307–1312 (2008).
Wu, X. et al. Genetic variation in the prostate stem cell antigen gene PSCA confers susceptibility to urinary bladder cancer. Nat. Genet. 41, 991–995 (2009).
Rothman, N. et al. A multi-stage genome-wide association study of bladder cancer identifies multiple susceptibility loci. Nat. Genet. 42, 978–984 (2010).
Cheng, S., Andrew, A. S., Andrews, P. C. & Moore, J. H. Complex systems analysis of bladder cancer susceptibility reveals a role for decarboxylase activity in two genome-wide association studies. BioData Min. 9, 40 (2016).
Mo, L. et al. Hyperactivation of Ha-ras oncogene, but not Ink4a/Arf deficiency, triggers bladder tumorigenesis. J. Clin. Invest. 117, 314–325 (2007).
van Oers, J. M. M. et al. Chromosome 9 deletions are more frequent than FGFR3 mutations in flat urothelial hyperplasias of the bladder. Int. J. Cancer 119, 1212–1215 (2006).
Chow, N. H. et al. Papillary urothelial hyperplasia is a clonal precursor to papillary transitional cell bladder cancer. Int. J. Cancer 89, 514–518 (2000).
Obermann, E. C. et al. Frequent genetic alterations in flat urothelial hyperplasias and concomitant papillary bladder cancer as detected by CGH, LOH, and FISH analyses. J. Pathol. 199, 50–57 (2003).
van Rhijn, B. W. G., Montironi, R., Zwarthoff, E. C., Jöbsis, A. C. & van der Kwast, T. H. Frequent FGFR3 mutations in urothelial papilloma. J. Pathol. 198, 245–251 (2002).
Zhang, Z. T., Pak, J., Shapiro, E., Sun, T. T. & Wu, X. R. Urothelium-specific expression of an oncogene in transgenic mice induced the formation of carcinoma in situ and invasive transitional cell carcinoma. Cancer Res. 59, 3512–3517 (1999).
Puzio-Kuter, A. M. et al. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev. 23, 675–680 (2009).
Gao, J. et al. p53 deficiency provokes urothelial proliferation and synergizes with activated Ha-ras in promoting urothelial tumorigenesis. Oncogene 23, 687–696 (2004).
Casey, R. G. et al. Diagnosis and management of urothelial carcinoma in situ of the lower urinary tract: a systematic review. Eur. Urol. 67, 876–888 (2015).
Spruck, C. H. et al. Two molecular pathways to transitional cell carcinoma of the bladder. Cancer Res. 54, 784–788 (1994).
McKenney, J. K., Desai, S., Cohen, C. & Amin, M. B. Discriminatory immunohistochemical staining of urothelial carcinoma in situ and non-neoplastic urothelium: an analysis of cytokeratin 20, p53, and CD44 antigens. Am. J. Surg. Pathol. 25, 1074–1078 (2001).
Jung, S. et al. The role of immunohistochemistry in the diagnosis of flat urothelial lesions: a study using CK20, CK5/6, P53, Cd138, and Her2/Neu. Ann. Diagn. Pathol. 18, 27–32 (2014).
Sfakianos, J. P. et al. The role of PTEN tumor suppressor pathway staining in carcinoma in situ of the bladder. Urol. Oncol. 32, 657–662 (2014).
Cheng, L. et al. Precise microdissection of human bladder carcinomas reveals divergent tumor subclones in the same tumor. Cancer 94, 104–110 (2002).
Sidransky, D. et al. Clonal origin bladder cancer. N. Engl. J. Med. 326, 737–740 (1992).
Hafner, C., Knuechel, R., Stoehr, R. & Hartmann, A. Clonality of multifocal urothelial carcinomas: 10 years of molecular genetic studies. Int. J. Cancer 101, 1–6 (2002).
Lamy, P. et al. Paired exome analysis reveals clonal evolution and potential therapeutic targets in urothelial carcinoma. Cancer Res. 76, 5894–5906 (2016).
Majewski, T. et al. Understanding the development of human bladder cancer by using a whole-organ genomic mapping strategy. Lab. Invest. 88, 694–721 (2008).
Kram, A. et al. Mapping and genome sequence analysis of chromosome 5 regions involved in bladder cancer progression. Lab. Invest. 81, 1039–1048 (2001).
Kim, M.-S. et al. Evidence for alternative candidate genes near RB1 involved in clonal expansion of in situ urothelial neoplasia. Lab. Invest. 86, 175–190 (2006).
Lee, S. et al. Forerunner genes contiguous to RB1 contribute to the development of in situ neoplasia. Proc. Natl Acad. Sci. USA 104, 13732–13737 (2007).
Volkmer, J.-P. et al. Three differentiation states risk-stratify bladder cancer into distinct subtypes. Proc. Natl Acad. Sci. USA 109, 2078–2083 (2012).
Van Batavia, J. et al. Bladder cancers arise from distinct urothelial sub-populations. Nat. Cell Biol. 16, 982–991 (2014).
Shin, K. et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 472, 110–114 (2011).
Shin, K. et al. Cellular origin of bladder neoplasia and tissue dynamics of its progression to invasive carcinoma. Nat. Cell Biol. 16, 469–478 (2014).
Chan, K. S. et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc. Natl Acad. Sci. USA 106, 14016–14021 (2009).
Nordentoft, I. et al. Mutational context and diverse clonal development in early and late bladder cancer. Cell Rep. 7, 1649–1663 (2014).
Balbás-Martínez, C. et al. Recurrent inactivation of STAG2 in bladder cancer is not associated with aneuploidy. Nat. Genet. 45, 1464–1469 (2013).
Hurst, C. D., Platt, F. M., Taylor, C. F. & Knowles, M. A. Novel tumor subgroups of urothelial carcinoma of the bladder defined by integrated genomic analysis. Clin. Cancer Res. 18, 5865–5877 (2012).
Knowles, M. A. & Hurst, C. D. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 15, 25–41 (2015). A recent review of the molecular features of bladder cancer (with more detailed information than provided in this Primer).
Lindgren, D. et al. Molecular characterization of early-stage bladder carcinomas by expression profiles, FGFR3 mutation status, and loss of 9q. Oncogene 25, 2685–2696 (2006).
Blaveri, E. et al. Bladder cancer stage and outcome by array-based comparative genomic hybridization. Clin. Cancer Res. 11, 7012–7022 (2005).
Billerey, C. et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am. J. Pathol. 158, 1955–1959 (2001).
di Martino, E., L’Hôte, C. G., Kennedy, W., Tomlinson, D. C. & Knowles, M. A. Mutant fibroblast growth factor receptor 3 induces intracellular signaling and cellular transformation in a cell type- and mutation-specific manner. Oncogene 28, 4306–4316 (2009).
Nakanishi, Y. et al. Mechanism of oncogenic signal activation by the novel fusion kinase FGFR3-BAIAP2L1. Mol. Cancer Ther. 14, 704–712 (2015).
Williams, S. V., Hurst, C. D. & Knowles, M. A. Oncogenic FGFR3 gene fusions in bladder cancer. Hum. Mol. Genet. 22, 795–803 (2013).
Jebar, A. H. et al. FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene 24, 5218–5225 (2005).
Platt, F. M. et al. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin. Cancer Res. 15, 6008–6017 (2009).
Lindgren, D. et al. Combined gene expression and genomic profiling define two intrinsic molecular subtypes of urothelial carcinoma and gene signatures for molecular grading and outcome. Cancer Res. 70, 3463–3472 (2010).
López-Knowles, E. et al. PIK3CA mutations are an early genetic alteration associated with FGFR3 mutations in superficial papillary bladder tumors. Cancer Res. 66, 7401–7404 (2006).
Sjödahl, G. et al. A systematic study of gene mutations in urothelial carcinoma; inactivating mutations in TSC2 and PIK3R1. PLoS ONE 6, e18583 (2011).
Taylor, C. F., Platt, F. M., Hurst, C. D., Thygesen, H. H. & Knowles, M. A. Frequent inactivating mutations of STAG2 in bladder cancer are associated with low tumour grade and stage and inversely related to chromosomal copy number changes. Hum. Mol. Genet. 23, 1964–1974 (2014).
Solomon, D. A. et al. Frequent truncating mutations of STAG2 in bladder cancer. Nat. Genet. 45, 1428–1430 (2013).
Gui, Y. et al. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat. Genet. 43, 875–878 (2011).
Mitra, A. P., Birkhahn, M. & Cote, R. J. p53 and retinoblastoma pathways in bladder cancer. World J. Urol. 25, 563–571 (2007).
Cairns, P. et al. Point mutation and homozygous deletion of PTEN/MMAC1 in primary bladder cancers. Oncogene 16, 3215–3218 (1998).
Askham, J. M. et al. AKT1 mutations in bladder cancer: identification of a novel oncogenic mutation that can co-operate with E17K. Oncogene 29, 150–155 (2010).
Ross, J. S. et al. A high frequency of activating extracellular domain ERBB2 (HER2) mutation in micropapillary urothelial carcinoma. Clin. Cancer Res. 20, 68–75 (2014).
Tomlinson, D. C., Baldo, O., Harnden, P. & Knowles, M. A. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J. Pathol. 213, 91–98 (2007).
Tomlinson, D. C. & Knowles, M. A. Altered splicing of FGFR1 is associated with high tumor grade and stage and leads to increased sensitivity to FGF1 in bladder cancer. Am. J. Pathol. 177, 2379–2386 (2010).
Tomlinson, D. C., L’Hôte, C. G., Kennedy, W., Pitt, E. & Knowles, M. A. Alternative splicing of fibroblast growth factor receptor 3 produces a secreted isoform that inhibits fibroblast growth factor-induced proliferation and is repressed in urothelial carcinoma cell lines. Cancer Res. 65, 10441–10449 (2005).
Tomlinson, D. C., Baxter, E. W., Loadman, P. M., Hull, M. A. & Knowles, M. A. FGFR1-induced epithelial to mesenchymal transition through MAPK/PLCγ/COX-2-mediated mechanisms. PLoS ONE 7, e38972 (2012).
Rampias, T. et al. A new tumor suppressor role for the Notch pathway in bladder cancer. Nat. Med. 20, 1199–1205 (2014).
Urakami, S. et al. Epigenetic inactivation of Wnt inhibitory factor-1 plays an important role in bladder cancer through aberrant canonical Wnt/β-catenin signaling pathway. Clin. Cancer Res. 12, 383–391 (2006).
Marsit, C. J. et al. Epigenetic inactivation of SFRP genes and TP53 alteration act jointly as markers of invasive bladder cancer. Cancer Res. 65, 7081–7085 (2005).
Kastritis, E. et al. Somatic mutations of adenomatous polyposis coli gene and nuclear β-catenin accumulation have prognostic significance in invasive urothelial carcinomas: evidence for Wnt pathway implication. Int. J. Cancer 124, 103–108 (2009).
Zhu, X., Kanai, Y., Saito, A., Kondo, Y. & Hirohashi, S. Aberrant expression of beta-catenin and mutation of exon 3 of the beta-catenin gene in renal and urothelial carcinomas. Pathol. Int. 50, 945–952 (2000).
Dudziec, E., Gogol-Döring, A., Cookson, V., Chen, W. & Catto, J. Integrated epigenome profiling of repressive histone modifications, DNA methylation and gene expression in normal and malignant urothelial cells. PLoS ONE 7, e32750 (2012).
Vallot, C. et al. A novel epigenetic phenotype associated with the most aggressive pathway of bladder tumor progression. J. Natl Cancer Inst. 103, 47–60 (2011).
Reinert, T. et al. Comprehensive genome methylation analysis in bladder cancer: identification and validation of novel methylated genes and application of these as urinary tumor markers. Clin. Cancer Res. 17, 5582–5592 (2011).
Wolff, E. M. et al. Unique DNA methylation patterns distinguish noninvasive and invasive urothelial cancers and establish an epigenetic field defect in premalignant tissue. Cancer Res. 70, 8169–8178 (2010).
Sánchez-Carbayo, M. Hypermethylation in bladder cancer: biological pathways and translational applications. Tumour Biol. 33, 347–361 (2012).
Ching, C. B. et al. HER2 gene amplification occurs frequently in the micropapillary variant of urothelial carcinoma: analysis by dual-color in situ hybridization. Mod. Pathol. 24, 1111–1119 (2011).
Van Allen, E. M. et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 4, 1140–1153 (2014).
Kim, J. et al. Somatic ERCC2 mutations are associated with a distinct genomic signature in urothelial tumors. Nat. Genet. 48, 600–606 (2016).
Aine, M., Eriksson, P., Liedberg, F., Sjödahl, G. & Höglund, M. Biological determinants of bladder cancer gene expression subtypes. Sci. Rep. 5, 10957 (2015). This paper provides an excellent analysis of data from recent subtyping studies of bladder cancer and demonstrates overlap of the groups defined.
Kim, J. et al. Invasive bladder cancer: genomic insights and therapeutic promise. Clin. Cancer Res. 21, 4514–4524 (2015).
Aine, M., Eriksson, P., Liedberg, F., Höglund, M. & Sjödahl, G. On molecular classification of bladder cancer: out of one, many. Eur. Urol. 68, 921–923 (2015).
Lerner, S. P. et al. Bladder cancer molecular taxonomy: summary from a consensus meeting. Bladder Cancer 2, 37–47 (2016).
Sjödahl, G. et al. A molecular taxonomy for urothelial carcinoma. Clin. Cancer Res. 18, 3377–3386 (2012). The only expression subtyping study to date that includes bladder tumours of all grades and stages.
Damrauer, J. S. et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc. Natl Acad. Sci. USA 111, 3110–3115 (2014).
Choi, W. et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 25, 152–165 (2014). Molecular subtypes of MIBC such as basal, luminal and TP53-like are defined and their clinical implications analysed.
Hedegaard, J. et al. Comprehensive transcriptional analysis of early-stage urothelial carcinoma. Cancer Cell 30, 27–42 (2016).
Aine, M. et al. Integrative epigenomic analysis of differential DNA methylation in urothelial carcinoma. Genome Med. 7, 23 (2015).
Sylvester, R. J. et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 49, 466–477 (2006).
Dyrskjøt, L. et al. Gene expression in the urinary bladder: a common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res. 64, 4040–4048 (2004).
Rebouissou, S. et al. CDKN2A homozygous deletion is associated with muscle invasion in FGFR3-mutated urothelial bladder carcinoma. J. Pathol. 227, 315–324 (2012).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Donsky, H., Coyle, S., Scosyrev, E. & Messing, E. M. Sex differences in incidence and mortality of bladder and kidney cancers: national estimates from 49 countries. Urol. Oncol. 32, 40.e23–40.e31 (2014).
Rebouissou, S. et al. EGFR as a potential therapeutic target for a subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Sci. Transl Med. 6, 244ra91 (2014).
Kardos, J. et al. Claudin-low bladder tumors are immune infiltrated and actively immune suppressed. JCI Insight 1, e85902 (2016).
Sjödahl, G. et al. Toward a molecular pathologic classification of urothelial carcinoma. Am. J. Pathol. 183, 681–691 (2013).
Dadhania, V. et al. Meta-analysis of the luminal and basal subtypes of bladder cancer and the identification of signature immunohistochemical markers for clinical use. EBioMedicine 12, 105–117 (2016).
Cohen, R. A. & Brown, R. S. Clinical practice. Microscopic hematuria. N. Engl. J. Med. 348, 2330–2338 (2003).
Bruyninckx, R., Buntinx, F., Aertgeerts, B. & Van Casteren, V. The diagnostic value of macroscopic haematuria for the diagnosis of urological cancer in general practice. Br. J. Gen. Pract. 53, 31–35 (2003).
Babjuk, M. et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur. Urol. 71, 447–461 (2016).
Liedberg, F. et al. Fast-track access to urologic care for patients with macroscopic haematuria is efficient and cost-effective: results from a prospective intervention study. Br. J. Cancer 115, 770–775 (2016).
Richards, K. A., Ham, S., Cohn, J. A. & Steinberg, G. D. Urinary tract infection-like symptom is associated with worse bladder cancer outcomes in the Medicare population: implications for sex disparities. Int. J. Urol. 23, 42–47 (2016).
Aaronson, D. S. et al. Meta-analysis: does lidocaine gel before flexible cystoscopy provide pain relief? BJU Int. 104, 506–509 (2009).
van der Aa, M. N. M. et al. Cystoscopy revisited as the gold standard for detecting bladder cancer recurrence: diagnostic review bias in the randomized, prospective CEFUB trial. J. Urol. 183, 76–80 (2010).
Passoni, N. M. et al. Concordance in biomarker status between bladder tumors at time of transurethral resection and subsequent radical cystectomy: results of a 5-year prospective study. Bladder Cancer 2, 91–99 (2016).
Yafi, F. A. et al. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol. Oncol. 33, 66.e25–66.e31 (2015).
Lotan, Y. Promises and challenges of fluorescence cystoscopy. Urol. Oncol. 33, 261–264 (2015).
Naitoa, S. et al. The Clinical Research Office of the Endourological Society (CROES) multicentre randomised trial of narrow band imaging–assisted transurethral resection of bladder tumour (TURBT) versus conventional white light imaging-assisted TURBT in primary non-muscle-invasive bladder cancer patients: trial protocol and 1-year results. Eur. Urol. 70, 506–515 (2016).
Palou, J. et al. Multivariate analysis of clinical parameters of synchronous primary superficial bladder cancer and upper urinary tract tumor. J. Urol. 174, 859–861 (2005).
Sobin, L., Gospodarowicz, M. K. & Wittekind, C. (eds) in TNM Classification of Malignant Tumors 7th edn 262–265 (Wiley-Blackwell, 2009).
van Rhijn, B. W. G. et al. Pathological stage review is indicated in primary pT1 bladder cancer. BJU Int. 106, 206–211 (2010).
Fritsche, H.-M. et al. Characteristics and outcomes of patients with clinical T1 grade 3 urothelial carcinoma treated with radical cystectomy: results from an international cohort. Eur. Urol. 57, 300–309 (2010).
Eble, J. N., Sauter, G., Epstein, J. I. & Sesterhenn, I. A. Pathology and genetics of tumours of the urinary system and male genital organs. IARChttps://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb7/BB7.pdf (2004).
Sylvester, R. J. et al. High-grade Ta urothelial carcinoma and carcinoma in situ of the bladder. Urology 66, 90–107 (2005).
May, M. et al. Prognostic accuracy of individual uropathologists in noninvasive urinary bladder carcinoma: a multicentre study comparing the 1973 and 2004 World Health Organisation classifications. Eur. Urol. 57, 850–858 (2010).
Lotan, Y. et al. Bladder cancer screening in a high risk asymptomatic population using a point of care urine based protein tumor marker. J. Urol. 182, 52–57 (2009).
Chou, R. & Dana, T. Screening adults for bladder cancer: a review of the evidence for the U.S. preventive services task force. Ann. Intern. Med. 153, 461–468 (2010).
Lotan, Y. Analysis of genetics to identify susceptibility to secondary malignancies in patients with bladder cancer. BJU Int. 118, 12–13 (2016).
Kamat, A. M. et al. Bladder cancer. Lancet 388, 2796–2810 (2016).
Alfred Witjes, J. et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur. Urol. 71, 462–475 (2017).
Lerner, S. P. Bladder cancer: ASCO endorses EAU muscle-invasive bladder cancer guidelines. Nat. Rev. Urol. 13, 440–441 (2016).
Power, N. E. & Izawa, J. Comparison of guidelines on non-muscle invasive bladder cancer (EAU, CUA, AUA, NCCN. NICE). Bladder Cancer 2, 27–36 (2016). This article summarizes and explains some of the important recommendations by major organizations regarding the management of NMIBC.
Chang, S. S. et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J. Urol. 196, 1021–1029 (2016).
Herr, H. W. Role of repeat resection in non-muscle-invasive bladder cancer. J. Natl Compr. Canc. Netw. 13, 1041–1046 (2015).
Divrik, R. T., Yildirim, U., Zorlu, F. & Ozen, H. The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumors of the bladder who received intravesical mitomycin: a prospective, randomized clinical trial. J. Urol. 175, 1641–1644 (2006).
Divrik, R. T., S¸ahin, A. F., Yildirim, Ü., Altok, M. & Zorlu, F. Impact of routine second transurethral resection on the long-term outcome of patients with newly diagnosed pT1 urothelial carcinoma with respect to recurrence, progression rate, and disease-specific survival: a prospective randomised clinical trial. Eur. Urol. 58, 185–190 (2010).
Burger, M. et al. Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: a meta-analysis of detection and recurrence based on raw data. Eur. Urol. 64, 846–854 (2013).
Daneshmand, S. et al. Hexaminolevulinate blue-light cystoscopy in non-muscle-invasive bladder cancer: review of the clinical evidence and consensus statement on appropriate use in the USA. Nat. Rev. Urol. 11, 589–596 (2014).
Karadeniz, M. S. et al. Bipolar versus monopolar resection of benign prostate hyperplasia: a comparison of plasma electrolytes, hemoglobin and TUR syndrome. Springerplus 5, 1739 (2016).
Osman, Y. & Harraz, A. M. A review comparing experience and results with bipolar versus monopolar resection for treatment of bladder tumors. Curr. Urol. Rep. 17, 21 (2016).
Böhle, A., Jocham, D. & Bock, P. R. Intravesical bacillus Calmette–Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J. Urol. 169, 90–95 (2003).
Sylvester, R. et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, Bacillus Calmette–Guérin, and Bacillus Calmette–Guérin plus isoniazid in patients with intermediate- and high-risk. Eur. Urol. 57, 766–773 (2010).
Lamm, D. L. et al. Maintenance bacillus Calmette–Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J. Urol. 163, 1124–1129 (2000). A landmark study of the role of BCG in the management of NMIBC.
Sylvester, R. J., van der Meijden, A. P. M. & Lamm, D. L. Intravesical bacillus Calmette–Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J. Urol. 168, 1964–1970 (2002).
Kamat, A. M. et al. Definitions, end points, and clinical trial designs for non-muscle-invasive bladder cancer: recommendations from the International Bladder Cancer Group. J. Clin. Oncol. 34, 1935–1944 (2016).
Yates, D. R. et al. Treatment options available for bacillus Calmette–Guérin failure in non-muscle-invasive bladder cancer. Eur. Urol. 62, 1088–1096 (2012).
Sylvester, R. J., Oosterlinck, W. & van der Meijden, A. P. M. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J. Urol. 171, 2186–2190 (2004).
Perlis, N. et al. Immediate post-transurethral resection of bladder tumor intravesical chemotherapy prevents non-muscle-invasive bladder cancer recurrences: an updated meta-analysis on 2548 patients and quality-of-evidence review. Eur. Urol. 64, 421–430 (2013).
Willis, D. L. et al. Micropapillary bladder cancer: current treatment patterns and review of the literature. Urol. Oncol. 32, 826–832 (2014).
Martin-Doyle, W., Leow, J.J., Orsola, A., Chang, S.L. & Bellmunt, J. Improving selection criteria for early cystectomy in high-grade t1 bladder cancer: a meta-analysis of 15,215 patients. J. Clin Oncol. 33, 643–650 (2015).
Novara, G. et al. Systematic review and cumulative analysis of perioperative outcomes and complications after robot-assisted radical cystectomy. Eur. Urol. 67, 376–401 (2015).
Yuh, B. et al. Systematic review and cumulative analysis of oncologic and functional outcomes after robot-assisted radical cystectomy. Eur. Urol. 67, 402–422 (2015).
Bochner, B. H. et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: a randomized clinical trial. Eur. Urol. 67, 1042–1050 (2015).
Smith, N. D. et al. The RAZOR (randomized open versus robotic cystectomy) trial: study design and trial update. BJU Int. 115, 198–205 (2015).
Kurpad, R., Woods, M. & Pruthi, R. Current status of robot-assisted radical cystectomy and intracorporeal urinary diversion. Curr. Urol. Rep. 17, 42 (2016).
Bruins, H. M. et al. The impact of the extent of lymphadenectomy on oncologic outcomes in patients undergoing radical cystectomy for bladder cancer: a systematic review. Eur. Urol. 66, 1065–1077 (2014).
World Health Organization (WHO) Consensus Conference on Bladder Cancer et al. Urinary diversion. Urology 69, 17–49 (2007).
Daneshmand, S. et al. Enhanced recovery protocol after radical cystectomy for bladder cancer. J. Urol. 192, 50–55 (2014).
Tyson, M. D. & Chang, S. S. Enhanced recovery pathways versus standard care after cystectomy: a meta-analysis of the effect on perioperative outcomes. Eur. Urol. 70, 995–1003 (2016).
Stein, J. P. et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J. Clin. Oncol. 19, 666–675 (2001).
Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data: advanced bladder cancer (ABC) meta-analysis collaboration. Eur. Urol. 48, 202–205 (2005). This meta-analysis is often cited to support neoadjuvant chemotherapy for patients with MIBC.
Yuh, B. E., Ruel, N., Wilson, T. G., Vogelzang, N. & Pal, S. K. Pooled analysis of clinical outcomes with neoadjuvant cisplatin and gemcitabine chemotherapy for muscle invasive bladder cancer. J. Urol. 189, 1682–1686 (2013).
Sternberg, C. N. et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol. 16, 76–86 (2015).
Leow, J. J. et al. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur. Urol. 66, 42–54 (2014).
Bayoumi, Y., Heikal, T. & Darweish, H. Survival benefit of adjuvant radiotherapy in stage III and IV bladder cancer: results of 170 patients. Cancer Manag. Res. 6, 459–465 (2014).
Solsona, E. et al. Feasibility of radical transurethral resection as monotherapy for selected patients with muscle invasive bladder cancer. J. Urol. 184, 475–480 (2010).
Lyons, M. D. & Smith, A. B. Surgical bladder-preserving techniques in the management of muscle-invasive bladder cancer. Urol. Oncol. 34, 262–270 (2016).
Knoedler, J. J. et al. Does partial cystectomy compromise oncologic outcomes for patients with bladder cancer compared to radical cystectomy? A matched case–control analysis. J. Urol. 188, 1115–1119 (2012).
Ploussard, G. et al. Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: a systematic review. Eur. Urol. 66, 120–137 (2014).
Bellmunt, J. & Petrylak, D. P. New therapeutic challenges in advanced bladder cancer. Semin. Oncol. 39, 598–607 (2012).
Alimohamed, N. S. & Sridhar, S. S. Options in metastatic urothelial cancer after first-line therapy. Curr. Opin. Support. Palliat. Care 9, 255–260 (2015).
Donin, N. M. et al. Immunotherapy in the treatment of urothelial carcinoma. J. Urol. 197, 14–22 (2017).
Svatek, R. S. et al. The economics of bladder cancer: costs and considerations of caring for this disease. Eur. Urol. 66, 253–262 (2014).
Ghosh, A. & Somani, B. K. Recent trends in postcystectomy health-related quality of life (QoL) favors neobladder diversion: systematic review of the literature. Urology 93, 22–26 (2016).
European Organisation for Research and Treatment of Cancer. Bladder Cancer: EORTC QLQ-NMIBC24, EORTC QLQ-BLM30. EORTChttp://groups.eortc.be/qol/bladder-cancer-eortc-qlq-nmibc24-eortc-qlq-blm30 (accessed 28 Feb 2017).
Blazeby, J. M. et al. Validation and reliability testing of the EORTC QLQ-NMIBC24 questionnaire module to assess patient-reported outcomes in non-muscle-invasive bladder cancer. Eur. Urol. 66, 1148–1156 (2014).
Anderson, C. B. et al. Psychometric characteristics of a condition-specific, health-related quality-of-life survey: the FACT-Vanderbilt Cystectomy Index. Urology 80, 77–83 (2012).
Gilbert, S. M. et al. Development and validation of the Bladder Cancer Index: a comprehensive, disease specific measure of health related quality of life in patients with localized bladder cancer. J. Urol. 183, 1764–1769 (2010).
Danna, B. J., Metcalfe, M. J., Wood, E. L. & Shah, J. B. Assessing symptom burden in bladder cancer: an overview of bladder cancer specific health-related quality of life instruments. Bladder Cancer 2, 329–340 (2016).
Melnyk, M., Casey, R. G., Black, P. & Koupparis, A. J. Enhanced recovery after surgery (ERAS) protocols: time to change practice? Can. Urol. Assoc. J. 5, 342–348 (2011).
Cerantola, Y. et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS®) society recommendations. Clin. Nutr. 32, 879–887 (2013).
Karl, A. et al. A new concept for early recovery after surgery for patients undergoing radical cystectomy for bladder cancer: results of a prospective randomized study. J. Urol. 191, 335–340 (2014).
Snyder, C. F. et al. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Qual. Life Res. 21, 1305–1314 (2012).
Basch, E. et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J. Clin. Oncol. 34, 557–565 (2016).
Nordlander, R., Hedman, A. & Pehrsson, S. K. Rate responsive pacing and exercise capacity — a comment. Pacing Clin. Electrophysiol. 12, 749–751 (1989).
Detmar, S. B., Muller, M. J., Schornagel, J. H., Wever, L. D. V. & Aaronson, N. K. Health-related quality-of-life assessments and patient–physician communication: a randomized controlled trial. JAMA 288, 3027–3034 (2002).
Velikova, G. et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J. Clin. Oncol. 22, 714–724 (2004).
Wu, A. W., Kharrazi, H., Boulware, L. E. & Snyder, C. F. Measure once, cut twice — adding patient-reported outcome measures to the electronic health record for comparative effectiveness research. J. Clin. Epidemiol. 66, S12–S20 (2013).
Davis, R. et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J. Urol. 188, 2473–2481 (2012).
Lotan, Y. et al. Prospective external validation of a bladder cancer detection model. J. Urol. 192, 1343–1348 (2014).
Loo, R. K. et al. Stratifying risk of urinary tract malignant tumors in patients with asymptomatic microscopic hematuria. Mayo Clin. Proc. 88, 129–138 (2013).
Mbeutcha, A., Lucca, I., Mathieu, R., Lotan, Y. & Shariat, S. F. Current status of urinary biomarkers for detection and surveillance of bladder cancer. Urol. Clin. North Am. 43, 47–62 (2016).
Kavalieris, L. et al. A segregation index combining phenotypic (clinical characteristics) and genotypic (gene expression) biomarkers from a urine sample to triage out patients presenting with hematuria who have a low probability of urothelial carcinoma. BMC Urol. 15, 23 (2015).
Godoy, G., Gakis, G., Smith, C. L. & Fahmy, O. Effects of androgen and estrogen receptor signaling pathways on bladder cancer initiation and progression. Bladder Cancer 2, 127–137 (2016). This comprehensive review describes the roles of the androgen and oestrogen signalling pathways in the initiation and progression of bladder cancer.
Dobruch, J. et al. Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur. Urol. 69, 300–310 (2016).
McGrath, M., Michaud, D. S. & De Vivo, I. Hormonal and reproductive factors and the risk of bladder cancer in women. Am. J. Epidemiol. 163, 236–244 (2005).
Daugherty, S. E. et al. Reproductive factors and menopausal hormone therapy and bladder cancer risk in the NIH-AARP Diet and Health Study. Int. J. Cancer 133, 462–472 (2013).
Izumi, K. et al. Expression of UDP-glucuronosyltransferase 1A in bladder cancer: association with prognosis and regulation by estrogen. Mol. Carcinog. 53, 314–324 (2014).
Takayama, K. et al. Identification of novel androgen response genes in prostate cancer cells by coupling chromatin immunoprecipitation and genomic microarray analysis. Oncogene 26, 4453–4463 (2007).
Izumi, K., Zheng, Y., Hsu, J.-W., Chang, C. & Miyamoto, H. Androgen receptor signals regulate UDP-glucuronosyltransferases in the urinary bladder: a potential mechanism of androgen-induced bladder carcinogenesis. Mol. Carcinog. 52, 94–102 (2013).
Izumi, K. et al. Androgen deprivation therapy prevents bladder cancer recurrence. Oncotarget 5, 12665–12674 (2015).
Mandel, P., Tilki, D. & Eslick, G. D. Extent of lymph node dissection and recurrence-free survival after radical cystectomy: a meta-analysis. Urol. Oncol. 32, 1184–1190 (2014).
Nix, J. et al. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. Eur. Urol. 57, 196–201 (2010).
Parekh, D. J., Messer, J., Fitzgerald, J., Ercole, B. & Svatek, R. Perioperative outcomes and oncologic efficacy from a pilot prospective randomized clinical trial of open versus robotic assisted radical cystectomy. J. Urol. 189, 474–479 (2013).
Fukuhara, H., Ino, Y. & Todo, T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 107, 1373–1379 (2016).
Adam, L. et al. Adenoviral mediated interferon-α 2b gene therapy suppresses the pro-angiogenic effect of vascular endothelial growth factor in superficial bladder cancer. J. Urol. 177, 1900–1906 (2007).
Dinney, C. P. N. et al. Phase I trial of intravesical recombinant adenovirus mediated interferon-α2b formulated in Syn3 for Bacillus Calmette–Guérin failures in nonmuscle invasive bladder cancer. J. Urol. 190, 850–856 (2013).
Navai, N. et al. Phase 1b Trial to evaluate tissue response to a second dose of intravesical recombinant adenoviral interferon α2b formulated in syn3 for failures of Bacillus Calmette–Guerin (BCG) therapy in nonmuscle invasive bladder cancer. Ann. Surg. Oncol. 23, 4110–4114 (2016).
Iyer, G. et al. Genome sequencing identifies a basis for everolimus sensitivity. Science 338, 221 (2012).
Lerner, S. P. et al. Summary and recommendations from the National Cancer Institute's clinical trials planning meeting on novel therapeutics for non-muscle invasive bladder cancer. Bladder Cancer 2, 165–202 (2016).
Rosenberg, J. E. et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387, 1909–1920 (2016). A pioneering study showing that a checkpoint inhibitor (atezolizumab) increases survival in chemotherapy-resistant advanced-stage bladder cancer.
Sacher, A. G. & Gandhi, L. Biomarkers for the clinical use of PD-1/PD-L1 inhibitors in non-small-cell lung cancer. JAMA Oncol. 2, 1217 (2016).
Allory, Y. et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur. Urol. 65, 360–366 (2014).
Hurst, C. D., Platt, F. M. & Knowles, M. A. Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine. Eur. Urol. 65, 367–369 (2014).
Simon, R. et al. Amplification pattern of 12q13-q15 genes (MDM2, CDK4, GLI) in urinary bladder cancer. Oncogene 21, 2476–2483 (2002).
Guo, G. et al. Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation. Nat. Genet. 45, 1459–1463 (2013).
Simon, R. et al. High-throughput tissue microarray analysis of 3p25 (RAF1) and 8p12 (FGFR1) copy number alterations in urinary bladder cancer. Cancer Res. 61, 4514–4519 (2001).
Cairns, P., Shaw, M. E. & Knowles, M. A. Initiation of bladder cancer may involve deletion of a tumour-suppressor gene on chromosome 9. Oncogene 8, 1083–1085 (1993).
Berggren, P. et al. p53 mutations in urinary bladder cancer. Br. J. Cancer 84, 1505–1511 (2001).
Wang, D. S. et al. Molecular analysis of PTEN and MXI1 in primary bladder carcinoma. Int. J. Cancer 88, 620–625 (2000).
Habuchi, T. et al. Oncogene amplification in urothelial cancers with p53 gene mutation or MDM2 amplification. J. Natl Cancer Inst. 86, 1331–1335 (1994).
Author information
Authors and Affiliations
Contributions
Introduction (O.S. and Y.L.); Epidemiology (J.D.); Mechanisms/pathophysiology (M.A.K.); Diagnosis, screening and prevention (M.B.); Management (M.A.); Quality of life (M.E.N.); Outlook (Y.L.); Overview of the Primer (O.S. and Y.L.).
Corresponding author
Ethics declarations
Competing interests
M.B. has received speaker honoraria from and consulted for Ipsen, Astellas Pharma, Janssen, Bayer and Takeda, and has received study grants from Ipsen and Janssen. Y.L. has served as a consultant for Photocure, Pacific Edge and MDxHealth and has conducted research studies with Abbott, Photocure, Cepheid, Pacific Edge and MDxHealth. All other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Sanli, O., Dobruch, J., Knowles, M. et al. Bladder cancer. Nat Rev Dis Primers 3, 17022 (2017). https://doi.org/10.1038/nrdp.2017.22
Published:
DOI: https://doi.org/10.1038/nrdp.2017.22
This article is cited by
-
Determining the clinicopathological significance of the VI-RADS ≧4 group: a retrospective study
BMC Urology (2024)
-
Tumorigenesis of basal muscle invasive bladder cancer was mediated by PTEN protein degradation resulting from SNHG1 upregulation
Journal of Experimental & Clinical Cancer Research (2024)
-
Investigating the impact of regulatory B cells and regulatory B cell-related genes on bladder cancer progression and immunotherapeutic sensitivity
Journal of Experimental & Clinical Cancer Research (2024)
-
Single-cell sequencing reveals the heterogeneity of B cells and tertiary lymphoid structures in muscle-invasive bladder cancer
Journal of Translational Medicine (2024)
-
EIF4A3-mediated biogenesis of circSTX6 promotes bladder cancer metastasis and cisplatin resistance
Journal of Experimental & Clinical Cancer Research (2024)