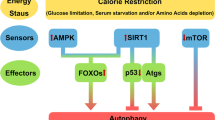
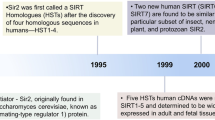
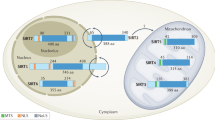
Abstract
Sirtuin 1 (SIRT1), the mammalian homolog of SIR2, was originally identified as a NAD-dependent histone deacetylase, the activity of which is closely associated with lifespan under calorie restriction. Growing evidence suggests that SIRT1 regulates glucose or lipid metabolism through its deacetylase activity for over two dozen known substrates, and has a positive role in the metabolic pathway through its direct or indirect involvement in insulin signaling. SIRT1 stimulates a glucose-dependent insulin secretion from pancreatic β cells, and directly stimulates insulin signaling pathways in insulin-sensitive organs. Furthermore, SIRT1 regulates adiponectin secretion, inflammatory responses, gluconeogenesis, and levels of reactive oxygen species, which together contribute to the development of insulin resistance. Moreover, overexpression of SIRT1 and several SIRT1 activators has beneficial effects on glucose homeostasis and insulin sensitivity in obese mice models. These findings suggest that SIRT1 might be a new therapeutic target for the prevention of disease related to insulin resistance, such as metabolic syndrome and diabetes mellitus, although direct evidence from clinical studies in humans is needed to prove this possibility. In this Review, we discuss the potential role and therapeutic promise of SIRT1 in insulin resistance on the basis of the latest experimental studies.
Key Points
-
SIRT1 upregulates adiponectin expression by deacetylating FoxO1, and thus protects against insulin resistance
-
SIRT1 attenuates tumor-necrosis-factor-induced inflammation, potentially by deacetylating NFκB
-
SIRT1 has a largely positive role in the insulin-signaling pathway by inducing insulin secretion, repressing Ptpn1, and by regulating insulin-receptor substrates (IRS) 1 and 2 and Akt activation
-
SIRT1 mediates mitochondrial biogenesis by deacetylating PGC1α, upregulates antioxidant enzyme expression by deacetylating FoxO3a and thereby reduces the levels of reactive oxygen species
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Newsholme, P. et al. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J. Physiol. 583, 9–24 (2007).
Muoio, D. M. & Newgard, C. B. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9, 193–205 (2008).
Kim, J. A., Wei, Y. & Sowers, J. R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 102, 401–414 (2008).
Rodgers, J. T. et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118 (2005).
Kitamura, Y. I. et al. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell. Metab. 2, 153–163 (2005).
Moynihan, K. A. et al. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell. Metab. 2, 105–117 (2005).
Picard, F. et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPARγ. Nature 429, 771–776 (2004).
Wang, H., Qiang, L. & Farmer, S. R. Identification of a domain within peroxisome proliferator-activated receptor gamma regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Mol. Cell Biol. 28, 188–200 (2008).
Rodgers, J. T. & Puigserver, P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc. Natl Acad. Sci. USA 104, 12861–12866 (2007).
Fulco, M. et al. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol. Cell. 12, 51–62 (2003).
Deng, X. Q., Chen, L. L. & Li, N. X. The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int. 27, 708–715 (2007).
Sommer, M. et al. δNp63α overexpression induces downregulation of Sirt1 and an accelerated aging phenotype in the mouse. Cell Cycle 5, 2005–2011 (2006).
Sun, C. et al. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B . Cell. Metab. 6, 307–319 (2007).
Milne, J. C. et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450, 712–716 (2007).
Feige, J. N. et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell. Metab. 8, 347–358 (2008).
Kadowaki, T. et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 116, 1784–1792 (2006).
Yamauchi, T. et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 7, 941–946 (2001).
Qiao, L. & Shao, J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J. Biol. Chem. 281, 39915–39924 (2006).
Frescas, D., Valenti, L. & Accilli, D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J. Biol. Chem. 280, 20589–20595 (2005).
Zhu, M. et al. Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp. Gerontol. 39, 1049–1059 (2004).
Qiang, L., Wang, H. & Farmer, S. R. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha. Mol. Cell Biol. 27, 4698–4707 (2007).
Banks, A. S. et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell. Metab. 8, 333–341 (2008).
Cai, D. et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 11, 183–190 (2005).
Yeung, F. et al. Modulation of NFκB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23, 2369–2380 (2004).
Yang, S. R. et al. Sirtuin regulates cigarette smoke induced proinflammatory mediators release via RelA/p65 NF-κB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L567–L576 (2006).
Csiszar, A. et al. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am. J. Physiol. Heart Circ. Physiol. 294, H2721–H2735 (2008).
Fontana, L. Neuroendocrine factors in the regulation of inflammation: excessive adiposity and calorie restriction. Exp. Gerontol. 44, 41–45 (2009).
Yoshizaki, T. et al. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol. Cell Biol. 29, 1363–1374 (2009).
Bordone, L. et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 4, e31 (2006).
Ramsey, K. M. et al. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell 7, 78–88 (2008).
Seely, B. L. et al. Protein tyrosine phosphatase 1B interacts with the activated insulin receptor. Diabetes 45, 1379–1385 (1996).
Goldstein, B. J., Bittner-Kowalczyk, A., White, M. F. & Harbeck, M. Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein. J. Biol. Chem. 275, 4283–4289 (2000).
Zhang, J. The direct involvement of Sirt1 in insulin-induced insulin receptor substrate-2 tyrosine phosphorylation. J. Biol. Chem. 282, 34356–34364 (2007).
Chen, I. Y. et al. Histone H2A.z is essential for cardiac myocyte hypertrophy but opposed by silent information regulator 2α. J. Biol. Chem. 281, 19369–19377 (2006).
Inoue, G., Cheatham, B., Emkey, R. & Kahn, C. R. Dynamics of insulin signaling in 3T3-L1 adipocytes. Differential compartmentalization and trafficking of insulin receptor substrate (IRS)-1 and IRS-2. J. Biol. Chem. 273, 11548–11555 (1998).
Tanno, M., Sakamoto, J., Miura, T., Shimamoto, K. & Horio, Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J. Biol. Chem. 282, 6823–6832 (2006).
Dowell, P., Otto, T. C., Adi, S. & Lane, M. D. Convergence of peroxisome proliferator-activated receptor gamma and Foxo1 signaling pathways. J. Biol. Chem. 278, 45485–45491 (2003).
Subauste, A. R. & Burant, C. F. Role of FoxO1 in FFA-induced oxidative stress in adipocytes. Am. J. Physiol. Endocrinol. Metab. 293, E159–E164 (2007).
Nakae, J. et al. The LXXLL motif of murine forkhead transcription factor FoxO1 mediates Sirt1-dependent transcriptional activity. J. Clin. Invest. 116, 2473–2483 (2006).
Liu, Y. et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature 456, 269–273 (2008).
Hou, X. et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 283, 20015–20026 (2008).
Pfluger, P. T., Herranz, D., Velasco-Miguel, S., Serrano, M. & Tschöp, M. H. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl Acad. Sci. USA 105, 9793–9798 (2008).
Nedachi, T., Kadotani, A., Ariga, M., Katagiri, H. & Kanzaki, M. Ambient glucose levels qualify the potency of insulin myogenic actions by regulating SIRT1 and FoxO3α in C2C12 myocytes. Am. J. Physiol. Endocrinol. Metab. 294, E668–E678 (2008).
Bouras, T. et al. SIRT1 deacetylation and repression of p300 involves lysine residues 1020/1024 within the cell cycle regulatory domain 1. J. Biol. Chem. 280, 10264–10276 (2005).
Michael, L. F. et al. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc. Natl Acad. Sci. USA 98, 3820–3825 (2001).
Gerhart-Hines, Z. et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J. 26, 1913–1923 (2006).
Finck, B. N. & Kelly, D. P. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J. Clin. Invest. 116, 615–622 (2006).
Nemoto, S., Fergusson, M. M. & Finkel, T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J. Biol. Chem. 280, 16456–16460 (2005).
Lagouge, M. et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127, 1109–1122 (2006).
Lin, J. et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 418, 797–801 (2002).
Anderson, R. M. et al. Dynamic regulation of PGC-1α localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress response. Aging Cell 7, 101–111 (2008).
Heilbronn, L. K., Gan, S. K., Turner, N., Campbell, L. V. & Chisholm, D. J. Markers of mitochondrial biogenesis and metabolism are lower in overweight and obese insulin-resistant subjects. J. Clin. Endocrinol. Metab. 92, 1467–1473 (2007).
Koves, T. R. et al. Peroxisome proliferator-activated receptor-gamma co-activator 1α-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J. Biol. Chem. 280, 33588–33598 (2005).
Koo, S. H. et al. PGC-1 promotes insulin resistance in liver through PAR-alpha-dependent induction of TRB-3. Nat. Med. 10, 530–534 (2004).
Nishikawa, T. & Araki, E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox. Signal. 9, 343–353 (2007).
Nishikawa, T. et al. Impact of mitochondrial ROS production in the pathogenesis of insulin resistance. Diabetes Res. Clin. Pract. 77 (Suppl. 1), S161–S164 (2007).
Guarente, L. Sirtuins in aging and disease. Cold Spring Harb. Symp. Quant. Biol. 72, 483–488 (2007).
Hasegawa, K. et al. Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem. Biophys. Res. Commun. 372, 51–56 (2008).
Suwa, M., Nakano, H., Radak, Z. & Kumagai, S. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor gamma coactivator-1α protein expressions in rat skeletal muscle. Metabolism 57, 986–998 (2008).
Elliott, P. J. & Jirousek, M. Sirtuins: novel targets for metabolic disease. Curr. Opin. Investig. Drugs 9, 371–378 (2008).
Acknowledgements
The authors express their sincere appreciation to Professor Ryuichi Kikkawa, Professor Masakazu Haneda, and Professor Atsunori Kashiwagi for their contribution to the discussion of the role of SIRT1 in insulin resistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Liang, F., Kume, S. & Koya, D. SIRT1 and insulin resistance. Nat Rev Endocrinol 5, 367–373 (2009). https://doi.org/10.1038/nrendo.2009.101
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2009.101
This article is cited by
-
Effects of Inorganic Arsenic on Type 2 Diabetes Mellitus In Vivo: the Roles and Mechanisms of miRNAs
Biological Trace Element Research (2024)
-
Tryptophanylation of insulin receptor by WARS attenuates insulin signaling
Cellular and Molecular Life Sciences (2024)
-
Treatment with recombinant Sirt1 rewires the cardiac lipidome and rescues diabetes-related metabolic cardiomyopathy
Cardiovascular Diabetology (2023)
-
Sirtuin1, not NAMPT, possesses anti-inflammatory effects in epicardial, pericardial and subcutaneous adipose tissue in patients with CHD
Journal of Translational Medicine (2023)
-
A preliminary study on the pathology and molecular mechanism of fumonisin B1 nephrotoxicity in young quails
Environmental Science and Pollution Research (2023)