Abstract
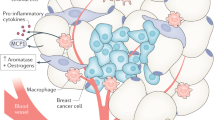
Adipose-tissue-derived signaling molecules, including the adipokines, are emerging as key candidate molecules that link obesity with cancer. Peritumoral, stromal, adipose tissue and secreted adipokines, particularly leptin, have important roles in breast cancer biology. For example, leptin signaling contributes to the metabolic features associated with breast cancer malignancy, such as switching the cells' energy balance from mitochondrial β-oxidation to the aerobic glycolytic pathway. Leptin also shapes the tumor microenvironment, mainly through its ability to potentiate both migration of endothelial cells and angiogenesis, and to sustain the recruitment of macrophages and monocytes, which in turn secrete vascular endothelial growth factor and proinflammatory cytokines. This article presents an overview of current knowledge on the involvement of leptin in the pathogenesis and progression of breast cancer, highlighted by human, in vitro and animal studies. Data are presented on the functional crosstalk between leptin and estrogen signaling, which further contributes to promotion of breast carcinogenesis. Finally, future perspectives and clinical applications in which leptin and the leptin receptor are considered as potential therapeutic targets for breast cancer are reviewed.
Key Points
-
Breast tumor development and progression is a long-term and continuous process that relies on reciprocal interactions between the tumor cell and its surrounding microenvironment
-
Leptin is one of the adipokines secreted by adipocytes—the most abundant cell type surrounding breast cancer cells—and is linked to breast cancer development
-
Breast cancer cells also produce leptin, which acts in an autocrine and paracrine manner to promote angiogenesis, tumor cell proliferation, migration and invasion, and has proinflammatory effects
-
Local estrogen production is increased by leptin, which amplifies estrogen signaling, further contributing to breast tumorigenesis
-
Leptin and leptin-receptor signaling might represent a potential therapeutic target for breast cancer treatment—particularly in women with obesity
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Calle, E. E., Rodriguez, C., Walker-Thurmond, K. & Thun, M. J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of, US adults. N. Engl. J. Med. 348, 1625–1638 (2003).
Calle, E. E. & Kaaks, R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 4, 579–591 (2004).
Lahmann, P. H. et al. Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer And Nutrition (EPIC). Int. J. Cancer 111, 762–771 (2004).
van den Brandt, P. A. et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am. J. Epidemiol. 152, 514–527 (2000).
Michels, K. B., Terry, K. L. & Willett, W. C. Longitudinal study on the role of body size in premenopausal breast cancer. Arch. Intern. Med. 166, 2395–2402 (2006).
Harvie, M., Hooper, L. & Howell, A. H. Central obesity and breast cancer risk: a systematic review. Obes. Rev. 4, 157–173 (2003).
Renehan, A. G., Tyson, M., Egger, M., Heller, R. F. & Zwahlen, M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371, 569–578 (2008).
Paz-Filho, G., Lim, E. L., Wong, M. L. & Licinio, J. Associations between adipokines and obesity-related cancer. Front. Biosci. 16, 1634–1650 (2011).
Witz, I. P. The tumor microenvironment—Introduction. Semin. Cancer Biology 12, 87–88 (2002).
Witz, I. P. The tumor microenvironment: the making of a paradigm. Cancer Microenviron. 1, 9–17 (2009).
Polyak, K. & Kalluri, R. The role of the microenvironment in mammary gland development and cancer. Cold Spring Harb. Perspect. Biol. 2, a003244 (2010).
Wiseman, B. S. & Werb, Z. Stromal effects on mammary gland development and breast cancer. Science 296, 1046–1049 (2002).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Coussens, L. M. & Werb, Z. Inflammatory cells and cancer: think different! J. Exp. Med. 193, F23–F26 (2001).
Coussens, L. M., Tinkle, C. L., Hanahan, D. & Werb, Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 103, 481–490 (2000).
Meng, L. et al. Tumor necrosis factor α and interleukin 11 secreted by malignant breast epithelial cells inhibit adipocyte differentiation by selectively down-regulating CCAAT/enhancer binding protein α and peroxisome proliferator-activated receptor γ: mechanism of desmoplastic reaction. Cancer Res. 61, 2250–2255 (2001).
Andarawewa, K. L. et al. Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell–adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res. 65, 10862–10871 (2005).
Iyengar, P. et al. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene 22, 6408–6423 (2003).
MacDougald, O. A. & Burant, C. F. The rapidly expanding family of adipokines. Cell Metab. 6, 159–161 (2007).
Ahima, R. S. & Flier, J. S. Leptin. Annu. Rev. Physiol. 62, 413–437 (2000).
Garofalo, C. & Surmacz, E. Leptin and cancer. J. Cell. Physiol. 207, 12–22 (2006).
Catalano, S. et al. Evidence that leptin through STAT and CREB signalling enhances cyclin D1 expression and promotes human endometrial cancer proliferation. J. Cell. Physiol. 218, 490–500 (2009).
Neville, M. C., McFadden, T. B. & Forsyth, I. Hormonal regulation of mammary differentiation and milk secretion. J. Mammary Gland Biol. Neoplasia 7, 49–66 (2002).
Cirillo, D., Rachiglio, A. M., La Montagna, R., Giordano, A. & Normanno, N. Leptin signaling in breast cancer: an overview. J. Cell. Biochem. 105, 956–964 (2008).
Zheng, Q. et al. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth and abrogates tumor initiating cell survival. Endocr. Relat. Cancer 18, 491–503 (2011).
Sweeney, G. Leptin signalling. Cell. Signal. 14, 655–663 (2002).
Ahima, R. S. & Osei, S. Y. Leptin signalling. Physiol. Behav. 81, 223–241 (2004).
Hu, X., Juneja, S. C., Maihle, N. J. & Cleary, M. P. Leptin—a growth factor in normal and malignant breast cells and for normal mammary gland development. J. Natl Cancer Inst. 94, 1704–1711 (2002).
Ray, A., Nkhata, K. J. & Cleary, M. P. Effects of leptin on human breast cancer cell lines in relationship to estrogen receptor and HER2 status. Int. J. Oncol. 30, 1499–1509 (2007).
Yin, N. et al. Molecular mechanisms involved in the growth stimulation of breast cancer cells by leptin. Cancer Res. 64, 5870–5875 (2004).
Saxena, N. K., Vertino, P. M., Anania, F. A. & Sharma, D. Leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to cyclin D1 promoter via activation of Stat3. J. Biol. Chem. 282, 13316–13325 (2007).
Laud, K., Gourdou, I., Pessemesse, L., Peyrat, J. P. & Djiane, J. Identification of leptin receptors in human breast cancer: functional activity in the T47-D breast cancer cell line. Mol. Cell. Endocrinol. 188, 219–226 (2002).
Okumura, M. et al. Leptin and high glucose stimulate cell proliferation in MCF-7 human breast cancer cells: reciprocal involvement of PKC-α and PPAR expression. Biochim. Biophys. Acta 1592, 107–116 (2002).
Somasundar, P., Yu, A. K., Vona-Davis, L. & Mc Fadden, D. W. Differential effects of leptin on cancer in vitro. J. Surg. Res. 113, 50–55 (2003).
Dieudonne, M. N. et al. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem. Biophys. Res. Commun. 293, 622–628 (2002).
Considine, R. V. et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 334, 292–295 (1996).
Orban, Z., Remaley, A. T., Sampson, M., Trajanoski, Z. & Chrousos, G. P. The differential effect of food intake and β-adrenergic stimulation on adipose-derived hormones and cytokines in man. J. Clin. Endocrinol. Metab. 84, 2126–2133 (1999).
Lee, W. M., Lu, S., Medline, A. & Archer, M. C. Susceptibility of lean and obese Zucker rats to tumorigenesis induced by N-methyl-N-nitrosurea. Cancer Lett. 162, 155–160 (2001).
Cleary, M. P. et al. Genetically obese MMTV-TGF-α/LepobLepob female mice do not develop mammary tumors. Breast Cancer Res. Treat 77, 205–215 (2003).
Cleary, M. P. et al. Leptin receptor-deficient MMTV-TGF-α/LeprdbLeprdb female mice do not develop oncogene-induced mammary tumors. Exp. Biol. Med. 229, 182–193 (2004).
Cleary, M. P., Grande, J. P. & Maihle, N. J. Effect of a high fat diet on body weight and mammary tumor latency in MMTV-TGF-α mice. Int. J. Obesity 28, 956–962 (2004).
Dogan, S. et al. Effects of high fat diet and/or body weight on mammary tumor leptin and apoptosis signalling pathways in MMTV-TGF-α mice. Breast Cancer Res. 9, R91 (2007).
Cleary, M. P., Grande, J. P., Juneja, S. C. & Maihle, N. J. Effect of dietary-induced obesity and mammary tumor development in MMTV-Neu female mice. Nutr. Cancer 50, 174–180 (2004).
Park, J., Kusminski, C. M., Chua, S. C. & Scherer, P. E. Leptin receptor signalling supports cancer cell metabolism through suppression of mitochondrial respiration in vivo. Am. J. Pathol. 177, 3133–3144 (2010).
De Luca, C. et al. Complete rescue of obesity, diabetes and infertility in db/db mice by neuron-specific LeprB transgenes. J. Clin. Invest. 115, 3484–3493 (2005).
Reich, N. C. STAT3 revs up the powerhouse. Sci. Signal. 2, pe61 (2009).
Gough, D. J. et al. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science 324, 1713–1716 (2009).
Wegrzyn, J. et al. Function of mitochondrial STAT3 in cellular respiration. Science 323, 793–797 (2009).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Sottnik, J. L., Lori, J. C., Rose, B. J. & Thamm, D. H. Glycolysis inhibition by 2-deoxy-D-glucose reverts the metastatic phenotype in vitro and in vivo. Clin. Exp. Metastasis doi:10.1007/s10585-011-9417-5.
Elstrom, R. L. et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 64, 3892–3899 (2004).
Plas, D. R. & Thompson, C. B. Akt-dependent transformation: there is more to growth than just surviving. Oncogene 24, 7435–7442 (2005).
Semenza, G. L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3, 721–732 (2003).
Gonzalez-Perez, R. R. et al. Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFκB/HIF-1α activation. Cell Signal 22, 1350–1362 (2010).
Sierra-Honigmann, M. R. et al. Biological action of leptin as an angiogenic factor. Science 281, 1683–1686 (1998).
Bouloumie, A., Drexler, H. C., Lafontan, M. & Busse, R. Leptin, the product of Ob gene, promotes angiogenesis. Circ. Res. 83, 1059–1066 (1998).
Goetze, S. et al. Leptin induces endothelial cell migration through Akt, which is inhibited by PPARγ-ligands. Hypertension 40, 748–754 (2002).
Friedl, P. & Wolf, K. Tumor cell invasion and migration: diversity and escape mechanism. Nat. Rev. Cancer 3, 362–374 (2003).
Morales-Ruiz, M. et al. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ. Res. 86, 892–896 (2000).
Dimmeler, S., Dernbach, E. &, Zeiher, A. M. Phosphorylation of the endothelial nitric oxide synthase at ser-1177 is required for VEGF-induced endothelial cell migration. FEBS Lett. 477, 258–262 (2000).
Papapetropoulos, A., Garcia-Cardena, G., Madri, J. A. & Sessa, W. C. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J. Clin. Invest. 100, 3131–3139 (1997).
Cho, S. Y. & Klemke, R. L. Extracellular-regulated kinase activation and CAS/Crk coupling regulate cell migration and suppress apoptosis during invasion of the extracellular matrix. J. Cell. Biol. 149, 223–236 (2000).
Zempo, N., Koyama, N., Kenagy, R. D., Lea, H. J. & Clowes, A. W. Regulation of vascular smooth muscle cell migration and proliferation in vitro and in injured rat arteries by a synthetic matrix metalloproteinase inhibitor. Arterioscler Thromb. Vasc. Biol. 16, 28–33 (1996).
Cho, A., Graves, J. & Reidy, M. A. Mitogen-activated protein kinases mediate matrix metalloproteinase-9 expression in vascular smooth muscle cells. Arterioscler Thromb. Vasc. Biol. 20, 2527–2532 (2000).
Park, H. Y. et al. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp. Mol. Med. 33, 95–102 (2001).
Gonzalez, R. R. et al. Leptin signalling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2). J. Biol. Chem. 281, 26320–26328 (2006).
Cao, R., Brakenhielm, E., Wahlestedt, C., Thyberg, J. & Cao, Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc. Natl Acad. Sci. USA 98, 6390–6395 (2001).
Zhou, W., Guo, S. & Gonzalez-Perez, R. R. Leptin proangiogenic signature in breast cancer is linked to IL-1 signalling. Br. J. Cancer 104, 128–137 (2011).
Wood, I. S., de Heredia, F. P., Wang, B. & Trayhurn, P. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc. Nutr. Soc. 68, 370–377 (2009).
Hirota, K. & Semenza, G. L. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit. Rev. Oncol. Hematol. 59, 15–26 (2006).
Ke, Q. & Costa, M. Hypoxia-inducible factor-1 (HIF-1). Mol. Pharmacol. 70, 1469–1480 (2006).
Cascio, S. et al. Mechanism of leptin expression in breast cancer cells: role of hypoxia-inducible factor-1α. Oncogene 27, 540–547 (2008).
Bartella, V. et al. Insulin-dependent leptin expression in breast cancer cells. Cancer Res. 68, 4919–4927 (2008).
Garofalo, C. et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin. Cancer Res. 12, 1447–1453 (2006).
Gruen, M. L., Hao, M., Piston, D. W. & Hasty, A. H. Leptin requires canonical migratory signalling pathways for induction of monocyte and macrophage chemotaxis. Am. J. Physiol. Cell Physiol. 293, C1481–C1488 (2007).
Leek, R. D. et al. Macrophage infiltration is associated with VEGF and EGFR expression in breast cancer. J. Pathol. 190, 430–436 (2000).
Bolat, F. et al. Microvessel density, VEGF expression, and tumor-associated macrophages in breast tumors: correlations with prognostic parameters. J. Exp. Clin. Cancer Res. 25, 365–372 (2006).
Santos-Alvarez, J., Goberna, R. & Sanchez-Margalet, V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell. Immunol. 194, 6–11 (1999).
Zarkesh-Esfahani, H. et al. Leptin indirectly activates human neutrophils via induction of TNFα. J. Immunol. 172, 1809–1814 (2004).
Maya-Monteiro, C. M. et al. Leptin induces macrophage lipid body formation by phosphatidylinositol 3-kinase- and mammalian target of rapamycin-dependent mechanism. J. Biol. Chem. 283, 2203–2210 (2008).
Maya-Monteiro, C. M. & Bozza, P. T. Leptin and mTOR. Partners in metabolism and inflammation. Cell Cycle 7, 1713–1717 (2008).
Larigauderie, G. et al. Adipophilin enhances lipid accumulation and prevents lipid efflux from THP-1 macrophages: potential role in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 24, 504–510 (2004).
Lindsley, J. E. & Rutter, J. Nutrient sensing and metabolic decisions. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 139, 543–559 (2004).
Wang, J., Liu, R., Hawkins, M., Barzilai, N. & Rossetti, L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 393, 684–688 (1998).
Roh, C., Han, J., Tzatsos, A. & Kandror, K. V. Nutrient-sensing mTOR-mediated pathway regulates leptin production in isolated rat adipocytes. Am. J. Physiol. Endocrinol. Metab. 284, 322–330 (2003).
Lynch, C. J. et al. Leucine in food mediates some of the postprandial rise in plasma leptin concentrations. Am. J. Physiol. Endocrinol. Metab. 291, E621–E630 (2006).
Grgor, M. F. & Hotamisligil, G. S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445 (2011).
Matarese, G., Procaccini, C., De Rosa, V., Horvath, T. L. & La Cava, A. Regulatory T cells in obesity: the leptin connection. Trends Mol. Med. 16, 247–256 (2010).
Vona-Davis, L. & Rose, D. P. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr. Relat. Cancer 14, 189–206 (2007).
Ishikawa, M., Kitayama, J. & Nagawa, H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin. Cancer Res. 10, 4325–4331 (2004).
Karaduman, M. et al. Tissue leptin levels in patients with breast cancer. J. BUON 15, 369–372 (2010).
Miyoshi, Y. et al. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int. J. Cancer 118, 1414–1419 (2006).
Révillion, F. et al. Messenger RNA expression of leptin and leptin receptors and their prognostic value in 322 human primary breast cancers. Clin. Cancer Res. 12, 2088–2094 (2006).
Jardé, T. et al. Leptin and leptin receptor involvement in cancer development: a study on human primary breast carcinoma. Oncol. Rep. 19, 905–911 (2008).
Simpson, E. R. et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 15, 342–355 (1994).
Yager, J. D. & Davidson, N. E. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 354, 270–282 (2006).
Bennett, P. A. et al. Differential expression and regulation of leptin receptor isoforms in the rat brain: effects of fasting and oestrogen. Neuroendocrinology 67, 29–36 (1998).
Machinal-Quelin, F., Dieudonne, M. N., Pecquery, R., Leneveu, M. C. & Giudicelli, Y. Direct in vitro effects of androgens and estrogens on ob gene expression and leptin secretion in human adipose tissue. Endocrine 18, 179–184 (2002).
Tessitore, L. et al. Adipocyte expression and circulating levels of leptin increase in both gynaecological and breast cancer patients. Int. J. Oncol. 24, 1529–1535 (2004).
Mauro, L. et al. Evidence that leptin up-regulates E-cadherin expression in breast cancer: effects on tumor growth and progression. Cancer Res. 67, 3412–3421 (2007).
Catalano, S. et al. Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor α in MCF-7 cells. J. Biol. Chem. 279, 19908–19915 (2004).
Catalano, S. et al. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J. Biol. Chem. 278, 28668–28676 (2003).
Garofalo, C., Sisci, D. & Surmacz, E. Leptin interferes with the effects of the antiestrogen ICI 182780 in MCF-7 breast cancer cells. Clin. Cancer Res. 10, 6466–6475 (2004).
Fusco, R. et al. Cellular and molecular crosstalk between leptin receptor and estrogen receptor-α in breast cancer: molecular basis for a novel therapeutic setting. Endocr. Relat Cancer 17, 373–382 (2010).
Binai, N. A. et al. Expression of estrogen receptor α increases leptin-induced STAT3 activity in breast cancer cells. Int. J. Cancer 127, 55–66 (2010).
Yu, W. et al. Regulation of estrogen receptors α and β in human breast carcinoma by exogenous leptin in nude mouse xenograft model. Chin. Med. J. (Engl.) 123, 337–343 (2010).
Magoffin, D. A., Weitsman, S. R., Aagarwal, S. K. & Jakimiuk, A. J. Leptin regulation of aromatase activity in adipose stromal cells from regularly cycling women. Ginekol Pol. 70, 1–7 (1999).
Lindell, K. et al. Leptin receptor 5′ untranslated regions in the rat: relative abundance, genomic organization and relation to putative response elements. Mol. Cell. Endocrinol. 172, 37–45 (2001).
Pasqualini, J. R. et al. Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and postmenopausal breast cancer patients. J. Clin. Endocrinol. Metab. 81, 1460–1464 (1996).
Geisler, J., Haynes, B., Ekse, D., Dowsett, M. & Lønning, P. E. Total body aromatization in postmenopausal breast cancer patients is strongly correlated to plasma leptin levels. J. Steroid Biochem. Mol. Biol. 104, 27–34 (2007).
Brown, K. A. et al. Subcellular localization of cyclic AMP-responsive element binding protein-regulated transcription coactivator 2 provides a link between obesity and breast cancer in postmenopausal women. Cancer Res. 69, 5392–5399 (2009).
Deb, S. et al. Estrogen regulates expression of tumor necrosis factor receptors in breast adipose fibroblasts. J. Clin. Endocrinol. Metab. 89, 4018–4024 (2004).
Schäffler, A., Schölmerich, J. & Buechler, C. Mechanisms if disease: adipokines and breast cancer- endocrine and paracrine mechainisms that connect adiposity and breast cancer. Nat. Clin. Pract Endocrinol. Metab. 3, 345–354 (2007).
Gertler, A. Development of leptin antagonists and their potential use in experimental biology and medicine. Trends Endocrinol. Metab. 17, 372–378 (2006).
Ray, A. & Cleary, M. P. Leptin as a potential therapeutic target for breast cancer prevention and treatment. Expert Opin. Ther. Targets. 14, 443–451 (2010).
Sandowski, Y. et al. Subcloning, expression, purification, and characterization of recombinant human leptin-binding domain. J. Biol. Chem. 277, 46304–46309 (2002).
Niv-Spector, L. et al. Mapping leptin-interacting sites in recombinant leptin-binding domain (LBD) subcloned from chicken leptin receptor. Biochem. J. 390, 475–484 (2005).
Otvos, L. et al. Development of a pharmacologically improved peptide agonist of the leptin receptor. Biochim. Biophys. Acta 1783, 1745–1754 (2008).
Otvos, L. et al. Peptide-based leptin receptor antagonists for cancer treatment and appetite regulation. Biopolymers 96, 117–125 (2011).
Otvos, L. et al. Efficacy of a leptin receptor antagonist peptide in a mouse model of triple-negative breast cancer. Eur. J. Cancer 47, 1578–1584 (2011).
Raver, N., Vardy, E., Livnah, O., Devos, R. & Gertler, A. Comparison of R128Q mutations in human, ovine, and chicken leptins. Gen. Comp. Endocrinol. 126, 52–58 (2002).
Gonzalez, R. R. & Leavis, P. C. A peptide derived from the human leptin molecule is a potent inhibitor of the leptin receptor function in rabbit endometrial cells. Endocrine 21, 185–195 (2003).
Gonzalez, R. R. et al. Leptin-signalling inhibition results in efficient antitumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Res. 11, R36 (2009).
Elinav, E. et al. Competitive inhibition of leptin signaling results in amelioration of liver fibrosis through modulation of stellate cell function. Hepatology 49, 278–286 (2009).
Elinav, E. et al. Pegylated leptin antagonist is a potent orexigenic agent: preparation and mechanism of activity. Endocrinology 150, 3083–3091 (2009).
Shpilman, M. et al. Development and characterization of high affinity leptins and leptin antagonists. J. Biol. Chem. 286, 4429–4442 (2011).
Catalano, S. et al. In vivo and in vitro evidence that PPARγ ligands are antagonists of leptin signaling in breast cancer. Am. J. Pathol. 179, 1030–1040 (2011).
Fazeli, M. et al. Identification of a monoclonal antibody against the leptin receptor that acts as an antagonist and blocks human monocyte and T cell activation. J. Immunol. Methods 312, 190–200 (2006).
Matarese, G. et al. Leptin increase in multiple sclerosis associates with reduced number of CD4+CD25+ regulatory T cells. Proc. Natl Acad. Sci. USA 102, 5150–5155 (2005).
De Rosa, V. et al. Leptin neutralization interferes with pathogenic T cell autoreactivity in autoimmune encephalomyelitis. J. Clin. Invest. 116, 447–455 (2006).
Jardé, T., Perrier, S., Vasson, M. P. & Caldefie-Chézet, F. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur. J. Cancer 47, 33–43 (2011).
Chen, D. C. et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 237, 109–114 (2006).
Nkhata, K. J., Ray, A., Dogan, S., Grande, J. P. & Cleary, M. P. Mammary tumor development from T47D human breast cancer cells in obese ovariectomized mice with and without estradiol supplements. Breast Cancer Res. Treat 114, 71–83 (2009).
Dogan, S., Rogozina, O. P., Loshkin, A., Grande, J. P. & Cleary, M. P. Effects of chronic vs intermittent calorie restriction on mammary tumor incidence and serum adiponectin and leptin levels in MMTV-TGF-α mice at different ages. Oncol. Lett. 1, 167–176 (2010).
Bullen, J. W., Bluher, S., Kelesidis, T. & Mantzoros, C. Regulation of adiponectin and its receptors in response to development of diet-induced obesity in mice. Am. J. Physiol. 292, E1079–E1086 (2007).
Varady, K. A., Allister, C. A., Roohk, D. J. & Hellerstein, M. K. Improvements in body fat distribution and circulating adiponectin by alternate-day fasting versus caloric restriction. J. Nutr. Biochem. 21, 188–195 (2009).
Miyazaki, Y. & DeFronzo, R. A. Rosiglitazone and pioglitazone similarly improve insulin sensitivity and secretion, glucose tolerance and adipocytokines in type 2 diabetic patients. Diabetes Obes. Metab. 10, 1204–1211 (2008).
Oz, O. et al. Arterial elasticity and plasma levels of adiponectin and leptin in type 2 diabetic patients treated with thiazolidinediones. Endocrine 33, 101–105 (2008).
Ferrara, A. et al. Cohort study of pioglitazone and cancer incidence in patients with diabetes. Diabetes Care 34, 923–929 (2011).
Schoonjans K and Auwerx, J. Thiazolidinediones: an update. Lancet 355, 1008–1010 (2000).
Sharma, P. K., Bhansali, A., Sialy, R., Malhotra, S. & Pandhi, P. Effects of pioglitazone and metformin on plasma adiponectin in newly detected type 2 diabetes mellitus. Clin. Endocrinol. 65, 722–728 (2006).
Steinberg, G. R., Macaulay, S. L., Febbraio, M. A. & Kemp, B. E. AMP-activated protein kinase—the fat controller of the energy railroad. Can. J. Physiol. Pharmacol. 84, 655–665 (2006).
Acknowledgements
S. Andò's and S. Catalano's research work is supported by Associazione Italiana per la Ricerca sul Cancro (AIRC) grant I G 1482.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the manuscript, including researching data for the article, writing and discussions of the content, as well as review and/or editing of the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Andò, S., Catalano, S. The multifactorial role of leptin in driving the breast cancer microenvironment. Nat Rev Endocrinol 8, 263–275 (2012). https://doi.org/10.1038/nrendo.2011.184
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2011.184
This article is cited by
-
Adipokines in atopic dermatitis: the link between obesity and atopic dermatitis
Lipids in Health and Disease (2024)
-
Reversion of breast epithelial polarity alterations caused by obesity
npj Breast Cancer (2023)
-
Identifying the effectiveness of 3D culture systems to recapitulate breast tumor tissue in situ
Cellular Oncology (2023)
-
Adipocytes secretome from normal and tumor breast favor breast cancer invasion by metabolic reprogramming
Clinical and Translational Oncology (2022)
-
Leptin signaling in breast cancer and its crosstalk with peroxisome proliferator-activated receptors α and γ
Clinical and Translational Oncology (2022)