Abstract
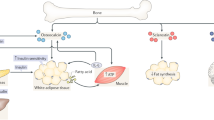
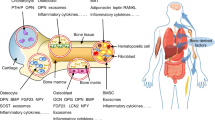
Increasing evidence supports an association between the skeleton and energy metabolism. These interactions are mediated by a variety of hormones, cytokines and nutrients. Here, the evidence for a role of osteocalcin in the regulation of glucose metabolism in humans is reviewed. Osteocalcin is a bone matrix protein that regulates hydroxyapatite size and shape through its vitamin-K-dependent, γ-carboxylated form. The concentration of osteocalcin in the circulation is a measure of bone formation. The undercarboxylated form of osteocalcin is active in glucose metabolism in mice. Total serum osteocalcin concentrations in humans are inversely associated with measures of glucose metabolism; however, human data are inconclusive with regard to the role of uncarboxylated osteocalcin in glucose metabolism because most studies do not account for the influence of vitamin K on the proportion of undercarboxylated osteocalcin or differentiate between the total and uncarboxylated forms of osteocalcin. Furthermore, most human studies do not concomitantly measure other bone turnover markers to isolate the role of osteocalcin as a measure of bone formation from its effect on glucose metabolism. Carefully designed studies are required to define the role of osteocalcin and its carboxylated or undercarboxylated forms in the regulation of glucose metabolism in humans.
Key Points
-
Osteocalcin is a calcium-binding bone matrix protein that contains the vitamin-K-dependent amino acid, γ-carboxyglutamic acid; circulating osteocalcin concentrations are a measure of bone formation
-
Studies in mice show that osteocalcin acts as a hormone to affect insulin sensitivity and energy expenditure; only the undercarboxylated form of osteocalcin is active
-
Human dietary intake of vitamin K is suboptimal, in contrast to that in mice—and, as a consequence, both bone and serum osteocalcin are undercarboxylated in humans
-
Most human studies examining the association between serum osteocalcin and measures of glucose metabolism do not differentiate between the total and undercarboxylated forms or take into account vitamin K intake
-
Most human studies also do not measure other bone turnover markers to distinguish circulating osteocalcin as a measure of bone turnover from its effect on glucose metabolism
-
In mice, the uncarboxylated form of osteocalcin is linked to glucose homeostasis, whereas in humans the data are inconclusive
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
23 November 2012
In the version of this article initially published online there was a mistake saying that binding of osteocalcin in vitro to a G-protein-coupled receptor (Gprc6a) in isolated mouse Leydig cells was associated with a reduction in the biosynthesis of testosterone. The sentence should have read "Furthermore, the researchers showed that binding of osteocalcin in vitro to a G-protein-coupled receptor (Gprc6a) in isolated mouse Leydig cells was associated with an increase in the biosynthesis of testosterone.79" The error has been corrected for the HTML and PDF versions of the article.
References
Stenflo, J., Fernlund, P., Egan, W. & Roepstorff, P. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proc. Natl Acad. Sci. USA 71, 2730–2733 (1974).
Suttie, J. W. The biochemical basis of warfarin therapy. Adv. Exp. Med. Biol. 214, 3–16 (1987).
Hauschka, P. V., Lian, J. B. & Gallop, P. M. Direct identification of the calcium-binding amino acid, γ-carboxyglutamate, in mineralized tissue. Proc. Natl Acad. Sci. USA 72, 3925–3929 (1975).
Price, P. A., Otsuka, A. A., Poser, J. W., Kristaponis, J. & Raman, N. Characterization of a γ-carboxyglutamic acid-containing protein from bone. Proc. Natl Acad. Sci. USA 73, 1447–1451 (1976).
Lee, N. K. et al. Endocrine regulation of energy metabolism by the skeleton. Cell 130, 456–469 (2007).
Ferron, M., Hinoi, E., Karsenty, G. & Ducy, P. Osteocalcin differentially regulates β cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl Acad. Sci. USA 105, 5266–5270 (2008).
Ferron, M. et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142, 296–308 (2010).
Fulzele, K. et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 142, 309–319 (2010).
Rosen, C. J. & Motyl, K. J. No bones about it: insulin modulates skeletal remodeling. Cell 142, 198–200 (2010).
Kumar, R. & Vella, A. Carbohydrate metabolism and the skeleton: picking a bone with the β-cell. J. Clin. Endocrinol. Metab. 96, 1269–1271 (2011).
Motyl, K. J., McCabe, L. R. & Schwartz, A. V. Bone and glucose metabolism: a two-way street. Arch. Biochem. Biophys. 503, 2–10 (2010).
Sullivan, T. R., Duque, G., Keech, A. C. & Herrmann, M. An old friend in a new light: the role of osteocalcin in energy metabolism. Cardiovasc. Ther. http://dx.doi.org/10.1111/j.1755-5922.2011.00300.x.
Reid, I. R. Relationships between fat and bone. Osteoporos. Int. 19, 595–606 (2007).
Kawai, M., Devlin, M. J. & Rosen, C. J. Fat targets for skeletal health. Nat. Rev. Rheumatol. 5, 365–372 (2009).
Karsenty, G. & Ferron, M. The contribution of bone to whole-organism physiology. Nature 481, 314–320 (2012).
Rosen, C. J. & Klibanski, A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am. J. Med. 122, 409–414 (2009).
Shearer, M. J., Fu, X. & Booth, S. L. Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv. Nutr. 3, 182–195 (2012).
Rishavy, M. A. & Berkner, K. L. Vitamin K oxygenation, glutamate carboxylation, and processivity: defining the three critical facets of catalysis by the vitamin K-dependent carboxylase. Adv. Nutr. 3, 135–148 (2012).
Berkner, K. L. The vitamin K-dependent carboxylase. Annu. Rev. Nutr. 25, 127–149 (2005).
Viegas, C. S. et al. Gla-rich protein (GRP), a new vitamin K-dependent protein identified from sturgeon cartilage and highly conserved in vertebrates. J. Biol. Chem. 283, 36655–36664 (2008).
Proudfoot, D. & Shanahan, C. M. Molecular mechanisms mediating vascular calcification: role of matrix Gla protein. Nephrology (Carlton) 11, 455–461 (2006).
Dowd, T. L., Rosen, J. F., Li, L. & Gundberg, C. M. The three-dimensional structure of bovine calcium ion-bound osteocalcin using 1H NMR spectroscopy. Biochemistry 42, 7769–7779 (2003).
Hauschka, P. V. & Carr, S. A. Calcium-dependent α-helical structure in osteocalcin. Biochemistry 21, 2538–2547 (1982).
Hoang, Q. Q., Sicheri, F., Howard, A. J. & Yang, D. S. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature 425, 977–980 (2003).
Desbois, C., Hogue, D. A. & Karsenty, G. The mouse osteocalcin gene cluster contains three genes with two separate spatial and temporal patterns of expression. J. Biol. Chem. 269, 1183–1190 (1994).
Kerner, S. A., Scott, R. A. & Pike, J. W. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3 . Proc. Natl Acad. Sci. USA 86, 4455–4459 (1989).
Lian, J. et al. Structure of the rat osteocalcin gene and regulation of vitamin D-dependent expression. Proc. Natl Acad. Sci. USA 86, 1143–1147 (1989).
Boivin, G et al. Localization of endogenous osteocalcin in neonatal rat bone and its absence in articular cartilage: effect of warfarin treatment. Virchows Arch. A. Pathol. Anat. Histopathol. 417, 505–512 (1990).
Price, P. A., Lothringer, J. W., Baukol, S. A. & Reddi, A. H. Developmental appearance of the vitamin K-dependent protein of bone during calcification. Analysis of mineralizing tissues in human, calf, and rat. J. Biol. Chem. 256, 3781–3784 (1981).
Hauschka, P. V. & Reid, M. L. Timed appearance of a calcium-binding protein containing γ-carboxyglutamic acid in developing chick bone. Dev. Biol. 65, 426–434 (1978).
Owen, T. A., et al. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J. Cell Physiol. 143, 420–430 (1990).
Ducy, P. et al. Increased bone formation in osteocalcin-deficient mice. Nature 382, 448–452 (1996).
Boskey, A. L. et al. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone 23, 187–196 (1998).
Poundarik, A., Gundberg, C. & Vashishth, D. Non-collageneous proteins influence bone mineral size, shape and orientation: a SAXS study. J. Bone Miner. Res. 26 (Suppl.), S36 (2011).
Fratzl, P., Paris, O., Klaushofer, K. & Landis, W. J. Bone mineralization in an osteogenesis imperfecta mouse model studied by small-angle X-ray scattering. J. Clin. Invest. 97, 396–402 (1996).
Brown, J. P., Delmas, P. D., Arlot, M. & Meunier, P. J. Active bone turnover of the cortico-endosteal envelope in postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 64, 954–959 (1987).
Brown, J. P. et al. Serum bone Gla-protein: a specific marker for bone formation in postmenopausal osteoporosis. Lancet 1, 1091–1093 (1984).
Eastell, R. et al. Bone formation rate in older normal women: concurrent assessment with bone histomorphometry, calcium kinetics, and biochemical markers. J. Clin. Endocrinol. Metab. 67, 741–748 (1988).
Price, P. A., Williamson, M. K. & Lothringer, J. W. Origin of the vitamin K-dependent bone protein found in plasma and its clearance by kidney and bone. J. Biol. Chem. 256, 12760–12766 (1981).
Garnero, P. et al. Measurement of serum osteocalcin with a human-specific two-site immunoradiometric assay. J. Bone Miner. Res. 7, 1389–1398 (1992).
Ivaska, K. K. et al. Release of intact and fragmented osteocalcin molecules from bone matrix during bone resorption in vitro. J. Biol. Chem. 279, 18361–18369 (2004).
Gundberg, C. M. & Weinstein, R. S. Multiple immunoreactive forms of osteocalcin in uremic serum. J. Clin. Invest. 77, 1762–1767 (1986).
Taylor, A. K. et al. Multiple osteocalcin fragments in human urine and serum as detected by a midmolecule osteocalcin radioimmunoassay. J. Clin. Endocrinol. Metab. 70, 467–472 (1990).
Ivaska, K. K. et al. Urinary osteocalcin as a marker of bone metabolism. Clin. Chem. 51, 618–628 (2005).
Eastell, R. & Hannon, R. A. Biomarkers of bone health and osteoporosis risk. Proc. Nutr. Soc. 67, 157–162 (2008).
Looker, A. C. et al. Clinical use of biochemical markers of bone remodeling: current status and future directions. Osteoporos. Int. 11, 467–480 (2000).
Price, P. A. & Williamson, M. K. Effects of warfarin on bone. Studies on the vitamin K-dependent protein of rat bone. J. Biol. Chem. 256, 12754–12759 (1981).
Benton, M. E., Price, P. A. & Suttie, J. W. Multi-site-specificity of the vitamin K-dependent carboxylase: in vitro carboxylation of des-γ-carboxylated bone Gla protein and des-γ-carboxylated pro bone Gla protein. Biochemistry 34, 9541–9551 (1995).
Cairns, J. R. & Price, P. A. Direct demonstration that the vitamin K-dependent bone Gla protein is incompletely γ-carboxylated in humans. J. Bone Miner. Res. 9, 1989–1997 (1994).
Booth, S. L. & Al Rajabi, A. Determinants of vitamin K status in humans. Vitam. Horm. 78, 1–22 (2008).
Food and Nutrition Board & Institute of Medicine. Dietary Reference Intakes for Vitamin A, vitamin K, Arsenic Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. (National Academy Press, Washington DC, 2001).
Suttie, J. W. The importance of menaquinones in human nutrition. Annu. Rev. Nutr. 15, 399–417 (1995).
Booth, S. L. et al. Dietary phylloquinone depletion and repletion in older women. J. Nutr. 133, 2565–2569 (2003).
Truong, J. T. et al. Age group and sex do not influence responses of vitamin K biomarkers to changes in dietary vitamin K. J. Nutr. 142, 936–941 (2012).
Booth, S. L. et al. Effect of vitamin K supplementation on bone loss in elderly men and women. J. Clin. Endocrinol. Metab. 93, 1217–1223 (2008).
Binkley, N. et al. Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density, or geometry in healthy postmenopausal North American women. J. Bone Miner. Res. 24, 983–991 (2009).
Cheung, A. M. et al. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO trial): a randomized controlled trial. PLoS Med. 5, e196 (2008).
Binkley, N. C., Krueger, D. C., Engelke, J. A., Foley, A. L. & Suttie, J. W. Vitamin K supplementation reduces serum concentrations of under-γ-carboxylated osteocalcin in healthy young and elderly adults. Am. J. Clin. Nutr. 72, 1523–1528 (2000).
Bolton-Smith, C. et al. Two-year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. J. Bone Miner. Res. 22, 509–519 (2007).
van Summeren, M. J. et al. The effect of menaquinone-7 (vitamin K2) supplementation on osteocalcin carboxylation in healthy prepubertal children. Br. J. Nutr. 102, 1171–1178 (2009).
Gundberg, C. M., Lian, J. B. & Booth, S. L. Vitamin K-dependent carboxylation of osteocalcin: friend or foe? Adv. Nutr. 3, 149–157 (2012).
Gundberg, C. M., Nieman, S. D., Abrams, S. & Rosen, H. Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J. Clin. Endocrinol. Metab. 83, 3258–3266 (1998).
Adami, S. Bone health in diabetes: considerations for clinical management. Curr. Med. Res. Opin. 25, 1057–1072 (2009).
Schwartz, A. V. et al. Older women with diabetes have an increased risk of fracture: a prospective study. J. Clin. Endocrinol. Metab. 86, 32–38 (2001).
Vestergaard, P., Rejnmark, L. & Mosekilde, L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia 48, 1292–1299 (2005).
Bonds, D. E. et al. Risk of fracture in women with type 2 diabetes: the Women's Health Initiative Observational Study. J. Clin. Endocrinol. Metab. 91, 3404–3410 (2006).
Vestergaard, P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos. Int. 18, 427–444 (2007).
Schwartz, A. V. et al. Intensive glycemic control is not associated with fractures or falls in the ACCORD randomized trial. Diabetes Care 35, 1525–1531 (2012).
Takada, I. et al. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-γ transactivation. Nat. Cell Biol. 9, 1273–1285 (2007).
Gimble, J. M. & Nuttall, M. E. The relationship between adipose tissue and bone metabolism. Clin. Biochem. 45, 874–879 (2012).
Griffith, J. F. et al. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology 236, 945–951 (2005).
Ducy, P. et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100, 197–207 (2000).
Elefteriou, F. Regulation of bone remodeling by the central and peripheral nervous system. Arch. Biochem. Biophys. 473, 231–236 (2008).
Hinoi, E. et al. The sympathetic tone mediates leptin's inhibition of insulin secretion by modulating osteocalcin bioactivity. J. Cell Biol. 183, 1235–1242 (2008).
Yip, S. C., Saha, S. & Chernoff, J. PTP1B: a double agent in metabolism and oncogenesis. Trends Biochem. Sci. 35, 442–449 (2010).
Zee, T., Settembre, C., Levine, R. L. & Karsenty, G. T-cell protein tyrosine phosphatase regulates bone resorption and whole-body insulin sensitivity through its expression in osteoblasts. Mol. Cell Biol. 32, 1080–1088 (2012).
Poser, J. W. & Price, P. A. A method for decarboxylation of γ-carboxyglutamic acid in proteins. Properties of the decarboxylated γ-carboxyglutamic acid protein from calf bone. J. Biol. Chem. 254, 431–436 (1979).
Kumm, J., Ivaska, K. K., Rohtla, K., Vaananen, K. & Tamm, A. Urinary osteocalcin and other markers of bone metabolism: the effect of risedronate therapy. Scand. J. Clin. Lab. Invest. 68, 459–463 (2008).
Oury, F. et al. Endocrine regulation of male fertility by the skeleton. Cell 144, 796–809 (2011).
Wellendorph, P. et al. Deorphanization of GPRC6A: a promiscuous L-α-amino acid receptor with preference for basic amino acids. Mol. Pharmacol. 67, 589–597 (2005).
Wellendorph, P. & Bräuner-Osborne, H. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene 335, 37–46 (2004).
Pi, M., Garner, S. C., Flannery, P., Spurney, R. F. & Quarles, L. D. Sensing of extracellular cations in CasR-deficient osteoblasts. Evidence for a novel cation-sensing mechanism. J. Biol. Chem. 275, 3256–3263 (2000).
Wellendorph, P. et al. No evidence for a bone phenotype in GPRC6A knockout mice under normal physiological conditions. J. Mol. Endocrinol. 42, 215–223 (2009).
Pi, M. et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS ONE 3, e3858 (2008).
Urakawa, I. et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444, 770–774 (2006).
Krakauer, J.C. et al. Bone loss and bone turnover in diabetes. Diabetes 44, 775–782 (1995).
Pater, A., Sypniewska, G. & Pilecki, O. Biochemical markers of bone cell activity in children with type 1 diabetes mellitus. J. Pediatr. Endocrinol. Metab. 23, 81–86 (2010).
Gerdhem, P., Isaksson, A., Akesson, K. & Obrant, K. J. Increased bone density and decreased bone turnover, but no evident alteration of fracture susceptibility in elderly women with diabetes mellitus. Osteoporos. Int. 16, 1506–1512 (2005).
Rosato, M. T., Schneider, S. H. & Shapses, S. A. Bone turnover and insulin-like growth factor I levels increase after improved glycemic control in noninsulin-dependent diabetes mellitus. Calcif. Tissue Int. 63, 107–111 (1998).
Bao, Y. Q. et al. Relationship between serum osteocalcin and glycaemic variability in type 2 diabetes. Clin. Exp. Pharmacol. Physiol. 38, 50–54 (2011).
Kanazawa, I. et al. Adiponectin is associated with changes in bone markers during glycemic control in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 94, 3031–3037 (2009).
Saleem, U., Mosley, T. H., Jr. & Kullo, I. J. Serum osteocalcin is associated with measures of insulin resistance, adipokine levels, and the presence of metabolic syndrome. Arterioscler. Thromb. Vasc Biol. 30, 1474–1478 (2010).
Pittas, A. G., Harris, S. S., Eliades, M., Stark, P. & Dawson-Hughes, B. Association between serum osteocalcin and markers of metabolic phenotype. J. Clin. Endocrinol. Metab. 94, 827–832 (2009).
Shea, M. K. et al. γ-Carboxylation of osteocalcin and insulin resistance in older men and women. Am. J. Clin. Nutr. 90, 1230–1235 (2009).
Hwang, Y. C., Jeong, I. K., Ahn, K. J. & Chung, H. Y. The uncarboxylated form of osteocalcin is associated with improved glucose tolerance and enhanced β-cell function in middle-aged male subjects. Diabetes Metab. Res. Rev. 25, 768–772 (2009).
Foresta, C. et al. Evidence for osteocalcin production by adipose tissue and its role in human metabolism. J. Clin. Endocrinol. Metab. 95, 3502–3506 (2010).
Shea, M. K. et al. Adulthood obesity is positively associated with adipose tissue concentrations of vitamin K and inversely associated with circulating indicators of vitamin K status in men and women. J. Nutr. 140, 1029–1034 (2010).
Pitroda, A. P., Harris, S. S. & Dawson-Hughes, B. The association of adiposity with parathyroid hormone in healthy older adults. Endocrine 36, 218–223 (2009).
Iki, M. et al. Serum undercarboxylated osteocalcin levels are inversely associated with glycemic status and insulin resistance in an elderly Japanese male population: Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Study. Osteoporos Int. 23, 761–770 (2012).
Gravenstein, K. S. et al. Cross-sectional evidence of a signaling pathway from bone homeostasis to glucose metabolism. J. Clin. Endocrinol. Metab. 96, E884–E890 (2011).
Boucher-Berry, C. et al. Vitamin D, osteocalcin and risk for adiposity as comorbidities in middle school children. J. Bone Miner. Res. 27, 283–293 (2012).
Misra, M. et al. Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. J. Clin. Endocrinol. Metab. 92, 2046–2052 (2007).
Pollock, N. K. et al. Lower uncarboxylated osteocalcin concentrations in children with prediabetes is associated with β-cell function. J. Clin. Endocrinol. Metab. 96, E1092–E1099 (2011).
Iglesias, P. et al. Serum concentrations of osteocalcin, procollagen type 1 N-terminal propeptide and β-CrossLaps in obese subjects with varying degrees of glucose tolerance. Clin. Endocrinol. 75, 184–188 (2011).
Fernández-Real, J. M. et al. The relationship of serum osteocalcin concentration to insulin secretion, sensitivity, and disposal with hypocaloric diet and resistance training. J. Clin. Endocrinol. Metab. 94, 237–245 (2009).
Bulló, M., Moreno-Navarrete, J. M., Fernández-Real, J. M. & Salas-Salvadó, J. Total and undercarboxylated osteocalcin predict changes in insulin sensitivity and β cell function in elderly men at high cardiovascular risk. Am. J. Clin. Nutr. 95, 249–255 (2012).
Schwartz, A. V. et al. Undercarboxylated osteocalcin does not predict development of diabetes in older adults in the Health, Aging and Body Composition Study. J. Bone Miner. Res. 26 (Suppl.) 69 (2011).
Lihn, A. S., Pedersen, S. B. & Richelsen, B. Adiponectin: action, regulation and association to insulin sensitivity. Obes. Rev. 6, 13–21 (2005).
Shi, Y. et al. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc. Natl Acad. Sci. USA 105, 20529–20533 (2008).
Lee, Y. J. et al. Serum osteocalcin is inversely associated with adipocyte-specific fatty acid-binding protein in the Korean metabolic syndrome research initiatives. Diabetes Care 33, e90 (2010).
Hamann, C. et al. Delayed bone regeneration and low bone mass in a rat model of insulin-resistant type 2 diabetes mellitus is due to impaired osteoblast function. Am. J. Physiol. Endocrinol. Metab. 301, E1220–E1228 (2011).
Maugeri, D. et al. Alendronate reduces the daily consumption of insulin (DCI) in patients with senile type I diabetes and osteoporosis. Arch. Gerontol. Geriatr. 34, 117–122 (2002).
Vestergaard, P. Risk of newly diagnosed type 2 diabetes is reduced in users of alendronate. Calcif. Tissue Int. 89, 265–270 (2011).
Keegan, T. H. et al. Effect of alendronate on bone mineral density and biochemical markers of bone turnover in type 2 diabetic women: the fracture intervention trial. Diabetes Care 27, 1547–1553 (2004).
Luckman, S. P. et al. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J. Bone Miner. Res. 13, 581–589 (1998).
Gluck, O. & Maricic, M. Skeletal and nonskeletal effects of raloxifene. Curr. Osteoporos. Rep. 1, 123–128 (2003).
Schafer, A. L. et al. Change in undercarboxylated osteocalcin is associated with changes in body weight, fat mass, and adiponectin: parathyroid hormone1–84 or alendronate therapy in postmenopausal women with osteoporosis (the PaTH study). J. Clin. Endocrinol. Metab. 96, E1982–1989 (2011).
Sokoll, L. J. et al. Changes in serum osteocalcin, plasma phylloquinone, and urinary γ-carboxyglutamic acid in response to altered intakes of dietary phylloquinone in human subjects. Am. J. Clin. Nutr. 65, 779–784 (1997).
Yoshida, M., Booth, S. L., Meigs, J. B., Saltzman, E. & Jacques, P. F. Phylloquinone intake, insulin sensitivity, and glycemic status in men and women. Am. J. Clin. Nutr. 88, 210–215 (2008).
Yoshida, M. et al. Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care 31, 2092–2096 (2008).
Beulens, J. W. et al. Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes. Diabetes Care 33, 1699–1705 (2010).
Erkkila, A. T. & Booth, S. L. Vitamin K intake and atherosclerosis. Curr. Opin. Lipidol. 19, 39–42 (2008).
Carter, P., Gray, L. J., Troughton, J., Khunti, K. & Davies, M. J. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ 341, c4229 (2010).
Sakamoto, N., Nishiike, T., Iguchi, H. & Sakamoto, K. Possible effects of one week vitamin K (menaquinone-4) tablets intake on glucose tolerance in healthy young male volunteers with different descarboxy prothrombin levels. Clin. Nutr. 19, 259–263 (2000).
Choi, H. J. et al. Vitamin K2 supplementation improves insulin sensitivity via osteocalcin metabolism: a placebo-controlled trial. Diabetes Care 34, e147 (2011).
Knapen, M. H. et al. Association of vitamin K status with adiponectin and body composition in healthy subjects: uncarboxylated osteocalcin is not associated with fat mass and body weight. Br. J. Nutr. 108, 1017–1024 (2011).
Kumar, R., Binkley, N. & Vella, A. Effect of phylloquinone supplementation on glucose homeostasis in humans. Am. J. Clin. Nutr. 92, 1528–1532 (2010).
Booth, S. L. & Mayer, J. Warfarin use and fracture risk. Nutr. Rev. 58, 20–22 (2000).
Cropp, J. S. & Bussey, H. I. A review of enzyme induction of warfarin metabolism with recommendations for patient management. Pharmacotherapy 17, 917–928 (1997).
Mega, J. L. A new era for anticoagulation in atrial fibrillation. N. Engl. J. Med. 365, 1052–1054 (2011).
Ferron, M., Wei, J., Yoshizawa, T., Ducy, P. & Karsenty, G. An ELISA-based method to quantify osteocalcin carboxylation in mice. Biochem. Biophys. Res. Commun. 397, 691–696 (2010).
Hetzel, P. G., Glanzmann, R., Hasler, P. W., Ladewick, A. & Bührer, C. Coumarin embryopathy in an extremely low birth weight infant associated with neonatal hepatitis and ocular malformations. Eur. J. Pediatr. 165, 358–360 (2006).
Scheen, A. J. Drug interactions of clinical importance with antihyperglycaemic agents: an update. Drug Saf. 28, 601–631 (2005).
Soon, D. et al. Effect of exenatide on the pharmacokinetics and pharmacodynamics of warfarin in healthy Asian men. J. Clin. Pharmacol. 46, 1179–1187 (2006).
Thyfault, J. P. & Booth, F. W. Lack of regular physical exercise or too much inactivity. Curr. Opin. Clin. Nutr. Metab. Care 14, 374–378 (2011).
Levinger, I. et al. The effect of acute exercise on undercarboxylated osteocalcin in obese men. Osteoporos. Int. 22, 1621–1626 (2011).
Fujimura, R. et al. Effect of resistance exercise training on bone formation and resorption in young male subjects assessed by biomarkers of bone metabolism. J. Bone Miner. Res. 12, 656–662 (1997).
Yarasheski, K. E., Campbell, J. A. & Kohrt, W. M. Effect of resistance exercise and growth hormone on bone density in older men. Clin. Endocrinol. (Oxf.) 47, 223–229 (1997).
Schroeder, E. T., Hawkins, S. A. & Jaque, S. V. Musculoskeletal adaptations to 16 weeks of eccentric progressive resistance training in young women. J. Strength Cond. Res. 18, 227–235 (2004).
Judge, J. O. et al. Home-based resistance training improves femoral bone mineral density in women on hormone therapy. Osteoporos. Int. 16, 1096–1108 (2005).
Adami, S. et al. Physical activity and bone turnover markers: a cross-sectional and a longitudinal study. Calcif. Tissue Int. 83, 388–392 (2008).
Booth, S. L. et al. Relationships between dietary intakes and fasting plasma concentrations of fat-soluble vitamins in humans. J. Nutr. 127, 587–592 (1997).
Rochefort, G. Y. et al. Osteocalcin—insulin relationship in obese children: a role for the skeleton in energy metabolism. Clin. Endocrinol. (Oxf.) 75, 265–270 (2011).
Ashcroft, F. M. & Rorsman, P. Diabetes mellitus and the β cell: the last ten years. Cell 148, 1160–1171 (2012).
Yoshikawa, Y. et al. Genetic evidence points to an osteocalcin-independent influence of osteoblasts on energy metabolism. J. Bone Miner. Res. 26, 2012–2025 (2011).
Wei, W. et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. Proc. Natl Acad. Sci. USA 109, 3143–3148 (2012).
Clowes, J. A., Khosla, S. & Eastell, R. Potential role of pancreatic and enteric hormones in regulating bone turnover. J. Bone Miner. Res. 20, 1497–1506 (2005).
US Department of Agriculture. USDA National Nutrient Database for Standard Reference, Release 24 [online], (2012).
Acknowledgements
The authors' research is supported by the US Department of Agriculture (USDA), Agricultural Research Service, under Cooperative Agreement No. 58-1950-7-707, and NIH grants DK69341, AG14759, AR38460 and P30 DK04735. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the USDA.
Author information
Authors and Affiliations
Contributions
S. L. Booth, A. Centi and C. Gundberg researched data for the article, provided a substantial contribution to discussions of its content, wrote the article and reviewed and/or edited the manuscript before submission. S. R. Smith provided a substantial contribution to discussions of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Cross-sectional Associations between Bone Turnover Markers and Glucose Metabolism and Adiposity in Non-Diabetic Children and Adults (DOC 175 kb)
Rights and permissions
About this article
Cite this article
Booth, S., Centi, A., Smith, S. et al. The role of osteocalcin in human glucose metabolism: marker or mediator?. Nat Rev Endocrinol 9, 43–55 (2013). https://doi.org/10.1038/nrendo.2012.201
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2012.201
This article is cited by
-
Vitamin K: a Potential Neuroprotective Agent
Revista Brasileira de Farmacognosia (2023)
-
Empagliflozin ameliorates liver fibrosis in NASH rat model via targeting hepatic NF-κB/SOX9/OPN signaling and osteocalcin level
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Effect of vitamin K2 administration on depression status in patients with polycystic ovary syndrome: a randomized clinical trial
BMC Women's Health (2022)
-
The effect of menaquinone-7 supplementation on dp-ucMGP, PIVKAII, inflammatory markers, and body composition in type 2 diabetes patients: a randomized clinical trial
Nutrition & Diabetes (2022)
-
Long-term implications of COVID-19 on bone health: pathophysiology and therapeutics
Inflammation Research (2022)