Abstract
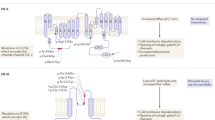
Primary aldosteronism is characterised by the dysregulation of aldosterone production and comprises both sporadic forms, caused by an aldosterone-producing adenoma or bilateral adrenal hyperplasia, and familial forms (familial hyperaldosteronism types I, II and III). The two principal physiological regulators of aldosterone synthesis are angiotensin II and serum K+, which reverse the high resting K+ conductance and hyperpolarized membrane potential of adrenal glomerulosa cells. The resulting membrane depolarization causes the opening of voltage-gated Ca2+ channels and an increase in intracellular Ca2+ that stimulates aldosterone biosynthesis. Point mutations in the KCNJ5 gene, which encodes the G-protein-activated inward rectifier K+ channel 4 (GIRK4), have been implicated in the pathogenesis of both sporadic and familial forms of primary aldosteronism. These mutations interfere with the selectivity filter of GIRK4 causing Na+ entry, cell depolarization and Ca2+ channel opening, resulting in constitutive aldosterone production. Seven families with familial hyperaldosteronism caused by KCNJ5 germline mutations have so far been described, and multicentre studies have reported KCNJ5 mutations in approximately 40% of sporadic aldosterone-producing adenomas. Herein, we review the role of GIRK4 in adrenal pathophysiology and provide an overview of the clinical and biochemical phenotypes resulting from KCNJ5 mutations in patients with sporadic and familial primary aldosteronism.
Key Points
-
Primary aldosteronism is the most frequent cause of secondary hypertension
-
Mutations in KCNJ5, which encodes the G-protein-activated inward rectifier K+ channel 4 GIRK4, cause familial hyperaldosteronism type III and around 40% of sporadic aldosterone-producing adenomas
-
KCNJ5 mutations alter the channel selectivity filter of the encoded K+ channel, which results in a calcium-mediated increase in aldosterone secretion
-
Most families with familial hyperaldosteronism type III display severe hyperaldosteronism with marked hypokalaemia from childhood and often require bilateral adrenalectomy
-
Patients with aldosterone-producing adenomas carrying KCNJ5 somatic mutations are more frequently female than those with wild-type KCNJ5
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
18 December 2012
In the version of this article initially published online there was a mistake in Figure 1: Na+ was incorrectly labelled as Na2+. The error has been corrected for the HTML and PDF versions of the article.
References
Funder, J. W. et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 93, 3266–3281 (2008).
Mulatero, P. et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J. Clin. Endocrinol. Metab. 89, 1045–1050 (2004).
Milliez, P. et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J. Am. Coll. Cardiol. 45, 1243–1248 (2005).
Catena, C. et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch. Intern. Med. 168, 80–85 (2008).
Fallo, F. et al. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J. Clin. Endocrinol. Metab. 91, 454–459 (2006).
Kempers, M. J. et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann. Intern. Med. 151, 329–337 (2009).
Sutherland, D. J., Ruse, J. L. & Laidlaw, J. C. Hypertension, increased aldosterone secretion and low plasma renin activity relieved by dexamethasone. Can. Med. Assoc. J. 95, 1109–1119 (1966).
Mulatero, P. et al. Prevalence and characteristics of familial hyperaldosteronism: the PATOGEN study (Primary Aldosteronism in TOrino—GENetic forms). Hypertension 58, 797–803 (2011).
Stowasser, M., Pimenta, E. & Gordon, R. D. Familial or genetic primary aldosteronism and Gordon syndrome. Endocrinol. Metab. Clin. North Am. 40, 343–368 viii (2011).
Lifton, R. P. et al. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature 355, 262–265 (1992).
Mulatero, P., Williams, T. A., Monticone, S. & Veglio, F. Is familial hyperaldosteronism underdiagnosed in hypertensive children? Hypertension 57, 1053–1055 (2011).
Pascoe, L. et al. Glucocorticoid-suppressible hyperaldosteronism and adrenal tumors occurring in a single French pedigree. J. Clin. Invest. 96, 2236–2246 (1995).
Pascoe, L. et al. Glucocorticoid-suppressible hyperaldosteronism results from hybrid genes created by unequal crossovers between CYP11B1 and CYP11B2. Proc. Natl Acad. Sci. USA 89, 8327–8331 (1992).
Litchfield, W. R. et al. Impaired potassium-stimulated aldosterone production: a possible explanation for normokalemic glucocorticoid-remediable aldosteronism. J. Clin. Endocrinol. Metab. 82, 1507–1510 (1997).
Dluhy, R. G., Anderson, B., Harlin, B., Ingelfinger, J. & Lifton, R. Glucocorticoid-remediable aldosteronism is associated with severe hypertension in early childhood. J. Pediatr. 138, 715–720 (2001).
Litchfield, W. R., Anderson, B. F., Weiss, R. J., Lifton, R. P. & Dluhy, R. G. Intracranial aneurysm and hemorrhagic stroke in glucocorticoid-remediable aldosteronism. Hypertension 31, 445–450 (1998).
Stowasser, M., Bachmann, A. W., Huggard, P. R., Rossetti, T. R. & Gordon, R. D. Severity of hypertension in familial hyperaldosteronism type I: relationship to gender and degree of biochemical disturbance. J. Clin. Endocrinol. Metab. 85, 2160–2166 (2000).
Mulatero, P. et al. Glucocorticoid remediable aldosteronism: low morbidity and mortality in a four-generation Italian pedigree. J. Clin. Endocrinol. Metab. 87, 3187–3191 (2002).
Fallo, F. et al. Coexistence of different phenotypes in a family with glucocorticoid-remediable aldosteronism. J. Hum. Hypertens. 18, 47–51 (2004).
Mulatero, P. et al. Recombinant CYP11B genes encode enzymes that can catalyze conversion of 11-deoxycortisol to cortisol, 18-hydroxycortisol, and 18-oxocortisol. J. Clin. Endocrinol. Metab. 83, 3996–4001 (1998).
Aglony, M. et al. Frequency of familial hyperaldosteronism type 1 in a hypertensive pediatric population: clinical and biochemical presentation. Hypertension 57, 1117–1121 (2011).
So, A. et al. Familial hyperaldosteronism type II is linked to the chromosome 7p22 region but also shows predicted heterogeneity. J. Hypertens. 23, 1477–1484 (2005).
Lafferty, A. R. et al. A novel genetic locus for low renin hypertension: familial hyperaldosteronism type II maps to chromosome 7 (7p22). J. Med. Genet. 37, 831–835 (2000).
Sukor, N. et al. Further evidence for linkage of familial hyperaldosteronism type II at chromosome 7p22 in Italian as well as Australian and South American families. J. Hypertens. 26, 1577–1582 (2008).
Newton-Cheh, C. et al. Clinical and genetic correlates of aldosterone-to-renin ratio and relations to blood pressure in a community sample. Hypertension 49, 846–856 (2007).
Geller, D. S. et al. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J. Clin. Endocrinol. Metab. 93, 3117–3123 (2008).
Choi, M. et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 331, 768–772 (2011).
Marionneau, C. et al. Specific pattern of ionic channel gene expression associated with pacemaker activity in the mouse heart. J. Physiol. 562, 223–234 (2005).
Mintert, E. et al. Generation of a constitutive Na+-dependent inward-rectifier current in rat adult atrial myocytes by overexpression of Kir3.4. J. Physiol. 585, 3–13 (2007).
Shankar, H. et al. Role of G protein-gated inwardly rectifying potassium channels in P2Y12 receptor-mediated platelet functional responses. Blood 104, 1335–1343 (2004).
Corey, S. & Clapham, D. E. Identification of native atrial G-protein-regulated inwardly rectifying K+ (GIRK4) channel homomultimers. J. Biol. Chem. 273, 27499–27504 (1998).
Wickman, K., Nemec, J., Gendler, S. J. & Clapham, D. E. Abnormal heart rate regulation in GIRK4 knockout mice. Neuron 20, 103–114 (1998).
Kovoor, P. et al. Evaluation of the role of I(KACh) in atrial fibrillation using a mouse knockout model. J. Am. Coll. Cardiol. 37, 2136–2143 (2001).
Perry, C. A. et al. Predisposition to late-onset obesity in GIRK4 knockout mice. Proc. Natl Acad. Sci. USA 105, 8148–8153 (2008).
Yang, Y. et al. Identification of a Kir3.4 mutation in congenital long QT syndrome. Am. J. Hum. Genet. 86, 872–880 (2010).
Monticone, S. et al. Effect of KCNJ5 mutations on gene expression in aldosterone-producing adenomas and adrenocortical cells. J. Clin. Endocrinol. Metab. 97, E1567–E1572 (2012).
Azizan, E. A. et al. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J. Clin. Endocrinol. Metab. 97, E819–E829 (2012).
Spät, A. Glomerulosa cell—a unique sensor of extracellular K+ concentration. Mol. Cell. Endocrinol. 217, 23–26 (2004).
Bandulik, S., Penton, D., Barhanin, J. & Warth, R. TASK1 and TASK3 potassium channels: determinants of aldosterone secretion and adrenocortical zonation. Horm. Metab. Res. 42, 450–457 (2010).
Condon, J. C., Pezzi, V., Drummond, B. M., Yin, S. & Rainey, W. E. Calmodulin-dependent kinase I regulates adrenal cell expression of aldosterone synthase. Endocrinology 143, 3651–3657 (2002).
Oki, K., Plonczynski, M. W., Lam, M. L., Gomez-Sanchez, E. P. & Gomez-Sanchez, C. E. The potassium channel, Kir3.4 participates in angiotensin II-stimulated aldosterone production by a human adrenocortical cell line. Endocrinology 153, 4328–4335 (2012).
McEwan, P. E., Vinson, G. P. & Kenyon, C. J. Control of adrenal cell proliferation by AT1 receptors in response to angiotensin II and low-sodium diet. Am. J. Physiol. 276, E303–E309 (1999).
Mulatero, P. A new form of hereditary primary aldosteronism: familial hyperaldosteronism type III. J. Clin. Endocrinol. Metab. 93, 2972–2974 (2008).
Mulatero, P. et al. KCNJ5 mutations in European families with nonglucocorticoid remediable familial hyperaldosteronism. Hypertension 59, 235–240 (2012).
Scholl, U. I. et al. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc. Natl Acad. Sci. USA 109, 2533–2538 (2012).
Charmandari, E. et al. A novel point mutation in the KCNJ5 gene causing primary hyperaldosteronism and early-onset autosomal dominant hypertension. J. Clin. Endocrinol. Metab. 97, E1532–1539 (2012).
Boulkroun, S. et al. Prevalence, clinical, and molecular correlates of KCNJ5 mutations in primary aldosteronism. Hypertension 59, 592–598 (2012).
Xekouki, P. et al. KCNJ5 mutations in the National Institutes of Health cohort of patients with primary hyperaldosteronism: an infrequent genetic cause of Conn's syndrome. Endocr. Relat. Cancer 19, 255–260 (2012).
Azizan, E. A. et al. Somatic mutations affecting the selectivity filter of KCNJ5 are frequent in 2 large unselected collections of adrenal aldosteronomas. Hypertension 59, 587–591 (2012).
Taguchi, R. et al. Expression and mutations of KCNJ5 mRNA in Japanese patients with aldosterone-producing adenomas. J. Clin. Endocrinol. Metab. 97, 1311–1319 (2012).
Akerström, T. et al. Comprehensive re-sequencing of adrenal aldosterone producing lesions reveal three somatic mutations near the KCNJ5 potassium channel selectivity filter. PLoS ONE 7, e41926 (2012).
Murthy, M., Azizan, E. A., Brown, M. J. & O'Shaughnessy, K. M. Characterization of a novel somatic KCNJ5 mutation delI157 in an aldosterone-producing adenoma. J. Hypertens. 30, 1827–1833 (2012).
Heitzmann, D. et al. Invalidation of TASK1 potassium channels disrupts adrenal gland zonation and mineralocorticoid homeostasis. EMBO J. 27, 179–187 (2008).
Davies, L. A. et al. TASK channel deletion in mice causes primary hyperaldosteronism. Proc. Natl Acad. Sci. USA 105, 2203–2208 (2008).
Niehrs, C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25, 7469–7481 (2006).
El Wakil, A. et al. Dkk3 is a component of the genetic circuitry regulating aldosterone biosynthesis in the adrenal cortex. Hum. Mol. Genet. 21, 4922–4929 (2012).
Nogueira, E. F. et al. The role of TASK1 in aldosterone production and its expression in normal adrenal and aldosterone-producing adenomas. Clin. Endocrinol. (Oxf). 73, 22–29 (2010).
Williams, T. A. et al. Teratocarcinoma-derived growth factor-1 is upregulated in aldosterone-producing adenomas and increases aldosterone secretion and inhibits apoptosis in vitro. Hypertension 55, 1468–1475 (2010).
Zennaro, M. C., Jeunemaitre, X. & Boulkroun, S. Integrating genetics and genomics in primary aldosteronism. Hypertension 60, 580–588 (2012).
Gomez-Sanchez, C. E. & Gomez-Sanchez, E. P. Mutations of the potassium channel KCNJ5 causing aldosterone-producing adenomas: one or two hits? Hypertension 59, 196–197 (2012).
Williams, T. A. et al. Visinin-like 1 is upregulated in aldosterone-producing adenomas with KCNJ5 mutations and protects from calcium-induced apoptosis. Hypertension 59, 833–839 (2012).
Braunewell, K. H. & Klein-Szanto, A. J. Visinin-like proteins (VSNLs): interaction partners and emerging functions in signal transduction of a subfamily of neuronal Ca2+-sensor proteins. Cell Tissue Res. 335, 301–316 (2009).
Mulatero, P. et al. 18-hydroxycorticosterone, 18-hydroxycortisol, and 18-oxocortisol in the diagnosis of primary aldosteronism and its subtypes. J. Clin. Endocrinol. Metab. 97, 881–889 (2012).
Bassett, M. H. et al. The orphan nuclear receptor NGFIB regulates transcription of 3beta-hydroxysteroid dehydrogenase. implications for the control of adrenal functional zonation. J. Biol. Chem. 279, 37622–37630 (2004).
Nogueira, E. F. & Rainey, W. E. Regulation of aldosterone synthase by activator transcription factor/cAMP response element-binding protein family members. Endocrinology 151, 1060–1070 (2010).
Ye, P., Nakamura, Y., Lalli, E. & Rainey, W. E. Differential effects of high and low steroidogenic factor-1 expression on CYP11B2 expression and aldosterone production in adrenocortical cells. Endocrinology 150, 1303–1309 (2009).
Oki, K., Plonczynski, M. W., Luis Lam, M., Gomez-Sanchez, E. P. & Gomez-Sanchez, C. E. Potassium channel mutant KCNJ5 T158A expression in HAC-15 cells increases aldosterone synthesis. Endocrinology 153, 1774–1782 (2012).
Hattangady, N. G., Olala, L. O., Bollag, W. B. & Rainey, W. E. Acute and chronic regulation of aldosterone production. Mol. Cell. Endocrinol. 350, 151–162 (2012).
Lu, L. et al. Nur-related factor 1 and nerve growth factor-induced clone B in human adrenal cortex and its disorders. J. Clin. Endocrinol. Metab. 89, 4113–4118 (2004).
Author information
Authors and Affiliations
Contributions
P. Mulatero and S. Monticone contributed equally to writing the article as joint first authors, and also researched data for the article and provided a substantial contribution to discussion of the content. W. Rainey and F. Veglio provided a substantial contribution to discussion of the content and reviewed and/or edited the manuscript before submission. T. A. Williams researched data for the article, provided a substantial contribution to discussion of the content and contributed to writing the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Mulatero, P., Monticone, S., Rainey, W. et al. Role of KCNJ5 in familial and sporadic primary aldosteronism. Nat Rev Endocrinol 9, 104–112 (2013). https://doi.org/10.1038/nrendo.2012.230
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2012.230
This article is cited by
-
Identifying primary aldosteronism patients who require adrenal venous sampling: a multi-center study
Scientific Reports (2023)
-
A rare case of hyporeninemic hypertension: Answers
Pediatric Nephrology (2021)
-
Pathogenesis and treatment of primary aldosteronism
Nature Reviews Endocrinology (2020)
-
GIRK1 triggers multiple cancer-related pathways in the benign mammary epithelial cell line MCF10A
Scientific Reports (2019)
-
Primary Aldosteronism: Cardiovascular Outcomes Pre- and Post-treatment
Current Cardiology Reports (2019)