Abstract
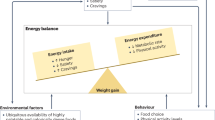
Obesity, which results from an imbalance between calorie intake and expenditure, now affects over 500 million individuals worldwide. Lifestyle and behavioural interventions aimed at reducing calorie intake and/or increasing energy expenditure have limited long-term effectiveness due to complex and persistent hormonal, metabolic and neurochemical adaptations that defend against weight loss and promote weight regain. Surgical treatments for obesity, although highly effective, are unavailable or unsuitable for the majority of individuals with excess adiposity. Accordingly, few effective treatment options are available to most individuals with obesity. In the past, the use of antiobesity drugs, seemingly the logical choice to fill this therapeutic gap, has been limited because of a lack of efficacy, poor long-term adherence rates and serious adverse effects. In 2012, the FDA approved two new medications—lorcaserin and phentermine–topiramate controlled release—and is currently reviewing the resubmission of naltrexone sustained release–bupropion sustained release. This Review presents the available data on the efficacy and safety of these three medications and discusses future perspectives and challenges related to pharmacological weight management.
Key Points
-
Lifestyle interventions for obesity rarely result in sustained weight loss and are generally characterized by high rates of recidivism or weight regain
-
The primary aim of pharmacological treatments for obesity is to suppress the biological drivers of weight gain and dampen the biological counter-response to weight loss
-
Emerging medications show promise for obtaining clinically relevant weight loss as well as improvements in comorbidities
-
Further studies are needed to assess the long-term benefits and cost-effectiveness of these new agents
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
WHO. Obesity and overweightfact sheet N.°311. WHO Media Centre [online], (2013).
Kaukua, J., Pekkarinen, T., Sane, T. & Mustajoki, P. Health-related quality of life in obese outpatients losing weight with very-low-energy diet and behaviour modification: a 2-y follow-up study. Int. J. Obes. Relat. Metab. Disord. 27, 1072–1080 (2003).
Cawley, J. & Meyerhoefer, C. The medical care costs of obesity: an instrumental variables approach. J. Health Econ. 31, 219–230 (2012).
Franz, M. J. et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J. Am. Diet Assoc. 107, 1755–1767 (2007).
Perry, B. & Wang, Y. Appetite regulation and weight control: the role of gut hormones. Nutr. Diabetes 2, e26 (2012).
Sumithran, P. et al. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 365, 1597–1604 (2011).
Goldsmith, R. et al. Effects of experimental weight perturbation on skeletal muscle work efficiency, fuel utilization, and biochemistry in human subjects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R79–R88 (2010).
Schwartz, M. W., Woods, S. C., Porte, D. Jr, Seeley, R. J. & Baskin, D. G. Central nervous system control of food intake. Nature 404, 661–671 (2000).
Dolgin, E. A history of drugs on the weight list. Nat. Med. 18, 843 (2012).
Vickers, S. P. & Dourish, C. T. 5-hydroxytryptamine receptor ligands and the treatment of obesity. Curr. Opin. Investig. Drugs 5, 377–388 (2004).
Bays, H. E. Lorcaserin and adiposopathy: 5-HT2c agonism as a treatment for 'sick fat' and metabolic disease. Expert Rev. Cardiovasc. Ther. 7, 1429–1445 (2009).
Connolly, H. M. et al. Valvular heart disease associated with fenfluramine–phentermine. N. Engl. J. Med. 337, 581–588 (1997).
Morton, G. J., Cummings, D. E., Baskin, D. G., Barsh, G. S. & Schwartz, M. W. Central nervous system control of food intake and body weight. Nature 443, 289–295 (2006).
Zhou, L. et al. 5-hydroxytryptamine 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signalling pathways. Cell Metab. 6, 398–405 (2007).
Smith, S. R. et al. Multicentre, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med. 363, 245–256 (2010).
Fidler, M. C. et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J. Clin. Endocrinol. Metab. 96, 3067–3077 (2011).
O'Neil, P. M. et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 20, 1426–1436 (2012).
Wilson, M. M. & Morley, J. E. Invited review: Aging and energy balance. J. Appl. Physiol. 95, 1728–1736 (2003).
Arena Pharmaceuticals. FDA Briefing Document NDA 22529 Lorqess (lorcaserin hydrochloride) tablets, 10 mg [online], (2010).
Haddock, C. K., Poston, W. S., Dill, P. L., Foreyt, J. P. & Ericsson, M. Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. Int. J. Obes. Relat. Metab. Disord. 26, 262–273 (2002).
Munro, J. F., MacCuish, A. C., Wilson, E. M. & Duncan, L. J. Comparison of continuous and intermittent anorectic therapy in obesity. Br. Med. J. 1, 352–354 (1968).
Phentermine in AHFS Drug Information (American Society of Health-System Pharmacists, 2005).
Silverstone, T. Appetite suppressants. A review. Drugs 43, 820–836 (1992).
Kim, K. K., Cho, H. J., Kang, H. C., Youn, B. B. & Lee, K. R. Effects on weight reduction and safety of short-term phentermine administration in Korean obese people. Yonsei Med. J. 47, 614–625 (2006).
Weintraub, M., Hasday, J. D., Mushlin, A. I. & Lockwood, D. H. A double-blind clinical trial in weight control. Use of fenfluramine and phentermine alone and in combination. Arch. Intern. Med. 144, 1143–1148 (1984).
Verrotti, A. et al. Topiramate-induced weight loss: a review. Epilepsy Res. 95, 189–199 (2011).
Kramer, C. K. et al. Efficacy and safety of topiramate on weight loss: a meta-analysis of randomized controlled trials. Obes. Rev. 12, e338–e347 (2011).
Wilding, J., Van Gaal, L., Rissanen, A., Vercruysse, F. & Fitchet, M. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int. J. Obes. Relat. Metab. Disord. 28, 1399–1410 (2004).
Roberts, M. Clinical Briefing Document. Endocrinologic and Metabolic Drugs Advisory Committee Meeting for phentermine/topiramate (Qnexa) [online], (2010).
Allison, D. B. et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring) 20, 330–342 (2012).
Gadde, K. M. et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 377, 1341–1352 (2011).
Garvey, W. T. et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am. J. Clin. Nutr. 95, 297–308 (2012).
Davidson, M. H. et al. Changes in cardiovascular risk associated with phentermine and topiramate extended-release in participants with comorbidities and a body mass index ≥27 kg/m2. Am. J. Cardiol. 111, 1131–1138 (2013).
Hunt, S. et al. Topiramate in pregnancy: preliminary experience from the UK Epilepsy and Pregnancy Register. Neurology 71, 272–276 (2008).
Bryant, S. G., Guernsey, B. G. & Ingrim, N. B. Review of bupropion. Clin. Pharm. 2, 525–537 (1983).
Goldstein, M. G. Bupropion sustained release and smoking cessation. J. Clin. Psychiatry 59 (Suppl. 4), 66–72 (1998).
Plodkowski, R. A. et al. Bupropion and naltrexone: a review of their use individually and in combination for the treatment of obesity. Expert Opin. Pharmacother. 10, 1069–1081 (2009).
Greenway, F. L. et al. Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring) 17, 30–39 (2009).
Cone, R. D. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 8, 571–578 (2005).
Reece, A. S. Hypothalamic opioid–melanocortin appetitive balance and addictive craving. Med. Hypotheses 76, 132–137 (2011).
Lobmaier, P. P., Kunoe, N., Gossop, M. & Waal, H. Naltrexone depot formulations for opioid and alcohol dependence: a systematic review. CNS Neurosci. Ther. 17, 629–636 (2011).
Greenway, F. L. et al. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J. Clin. Endocrinol. Metab. 94, 4898–4906 (2009).
Billes, S. K. & Greenway, F. L. Combination therapy with naltrexone and bupropion for obesity. Expert Opin. Pharmacother. 12, 1813–1826 (2011).
Maggio, C. A. et al. Naltrexone and human eating behaviour: a dose-ranging inpatient trial in moderately obese men. Brain Res. Bull. 14, 657–661 (1985).
Greenway, F. L. et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 376, 595–605 (2010).
Wadden, T. A. et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behaviour modification: the COR-BMOD trial. Obesity (Silver Spring) 19, 110–120 (2011).
Orexigen Therapeutics Inc. FDA Briefing Document NDA 200063 [online], (2010).
Crosby, R. D., Kolotkin, R. L. & Williams, G. R. An integrated method to determine meaningful changes in health-related quality of life. J. Clin. Epidemiol. 57, 1153–1160 (2004).
Padwal, R., Kezouh, A., Levine, M. & Etminan, M. Long-term persistence with orlistat and sibutramine in a population-based cohort. Int. J. Obes. (Lond.) 31, 1567–1570 (2007).
Bray, G. A. & Ryan, D. H. Drug treatment of obesity. Psychiatr. Clin. North Am. 34, 871–880 (2011).
Padwal, R. S., Pajewski, N. M., Allison, D. B. & Sharma, A. M. Using the Edmonton obesity staging system to predict mortality in a population-representative cohort of people with overweight and obesity. CMAJ 183, E1059–E1066 (2011).
Committee on National Statistics & Behavioural and Social Sciences and Education. The Prevention and Treatment of Missing Data in Clinical Trials (National Academies Press, 2010).
Acknowledgements
C. F. Rueda-Clausen's research is supported by the Canadian Institute of Health Research and Alberta Innovates Health Solutions. A. M. Sharma's and R. S. Padwal's research is supported by an alternative funding plan from the Government of Alberta, Canada, and the University of Alberta, Canada. A. M. Sharma is the Alberta Health Services Chair in Obesity Research and Management.
Author information
Authors and Affiliations
Contributions
C. F. Rueda-Clausen researched the data for the article. C. F. Rueda-Clausen, R. S. Padwal and A. M. Sharma contributed substantially to discussions of content, writing, review and/or editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
R. S. Padwal has acted as a consultant for VIVUS Pharmaceutical. A. M. Sharma has acted as a consultant for Arena Pharmaceutical, Orexigen Therapeutics and VIVUS Pharmaceutical in relation to their antiobesity drug programs. C. F. Rueda-Clausen declares no competing interests.
Rights and permissions
About this article
Cite this article
Rueda-Clausen, C., Padwal, R. & Sharma, A. New pharmacological approaches for obesity management. Nat Rev Endocrinol 9, 467–478 (2013). https://doi.org/10.1038/nrendo.2013.113
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2013.113
This article is cited by
-
Cholecystokinin-induced satiety, a key gut servomechanism that is affected by the membrane microenvironment of this receptor
International Journal of Obesity Supplements (2016)
-
Liraglutide 3.0 mg for Weight Management: A Population Pharmacokinetic Analysis
Clinical Pharmacokinetics (2016)
-
Antiobesity Strategy Targets Energy Economy Safeguards
Molecular Therapy (2015)
-
Next Generation of Weight Management Medications: Implications for Diabetes and CVD Risk
Current Cardiology Reports (2015)
-
Obesity Education Strategies for Cancer Prevention in Women’s Health
Current Obstetrics and Gynecology Reports (2015)