Key Points
-
Osteoporosis is a common disease that is characterized by an increased propensity to fracture owing to decreased bone mass and bone quality. Clinically, osteoporosis is diagnosed when a patient presents with a fracture that has resulted from minimal trauma; however, osteoporosis is commonly diagnosed, for the purposes of preventive therapy, through measurement of bone mineral density (BMD).
-
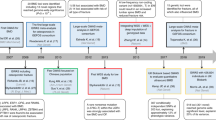
Genome-wide association studies (GWASs) have been performed for BMD using various strategies, such as multi-ethnic studies, using extreme phenotypes and large-scale meta-analyses. Although the loci identified in these studies have been repeated between studies, the effect sizes are small, explaining the lack of amenability of the genetics of osteoporosis to genetic-linkage studies.
-
GWASs for fracture have also been carried out: fracture has a lower heritability than BMD and decreases with age. The findings from GWASs are in their preliminary stages, and the genetics of fracture risk is still poorly understood, although higher-powered studies and those targeting the most heritable age range of fractures will result in further understanding.
-
Highlighting proteins on shared pathways by GWASs for BMD and fracture have furthered insights into the pathophysiology of osteoporosis. Pathways highlighted include the WNT pathway, the RANK–RANKL–OPG pathway and genes that are involved in enochodral ossification.
-
Genes highlighted by GWASs include known drug targets for therapies effective for treatment of osteoporosis. Furthermore, the GWAS hits at present have little demonstrated use in predicting who will experience fractures.
-
Future highly powered GWASs will elucidate further variants that will be informative for osteoporosis physiology and treatment.
Abstract
Osteoporosis is among the most common and costly diseases and is increasing in prevalence owing to the ageing of our global population. Clinically defined largely through bone mineral density, osteoporosis and osteoporotic fractures have reasonably high heritabilities, prompting much effort to identify the genetic determinants of this disease. Genome-wide association studies have recently provided rapid insights into the allelic architecture of this condition, identifying 62 genome-wide-significant loci. Here, we review how these new loci provide an opportunity to explore how the genetics of osteoporosis can elucidate its pathophysiology, provide drug targets and allow for prediction of future fracture risk.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
07 August 2012
In table 2 of the above article, the P value for locus 7q31.31 should have been 7.3 × 10−9. Two citations to table 2 in the sections ‘GWASs for fracture’ and ‘Insights into pathophysiology’ were incorrectly cited as table 1. In the section ‘GWASs for fracture’, two reference citations were incorrectly cited as 32: they should have been 74 and 31, respectively. The blurb for reference 31 should have read 'Together with reference 32'. The authors and editors apologize for these errors.
References
Kanis, J. A. Diagnosis of osteoporosis. Osteoporos. Int. 7 (Suppl. 3), S108–S116 (1997).
Burge, R. et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 22, 465–475 (2007).
Kanis, J. A., Johnell, O., Oden, A., Johansson, H. & McCloskey, E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos. Int. 19, 385–397 (2008).
Slemenda, C. W. et al. The genetics of proximal femur geometry, distribution of bone mass and bone mineral density. Osteoporos. Int. 6, 178–182 (1996).
Smith, D. M., Nance, W. E., Kang, K. W., Christian, J. C. & Johnston, C. C. Jr. Genetic factors in determining bone mass. J. Clin. Invest. 52, 2800–2808 (1973).
Arden, N. K., Baker, J., Hogg, C., Baan, K. & Spector, T. D. The heritability of bone mineral density, ultrasound of the calcaneus and hip axis length: a study of postmenopausal twins. J. Bone Miner. Res. 11, 530–534 (1996).
Marshall, D., Johnell, O. & Wedel, H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312, 1254–1259 (1996).
Jergas, M. & Gluer, C. C. Assessment of fracture risk by bone density measurements. Semin. Nucl. Med. 27, 261–275 (1997).
Andrew, T., Antioniades, L., Scurrah, K. J., Macgregor, A. J. & Spector, T. D. Risk of wrist fracture in women is heritable and is influenced by genes that are largely independent of those influencing BMD. J. Bone Miner. Res. 20, 67–74 (2005).
Michaelsson, K., Melhus, H., Ferm, H., Ahlbom, A. & Pedersen, N. L. Genetic liability to fractures in the elderly. Arch. Intern. Med. 165, 1825–1830 (2005).
Kaufman, J. M. et al. Genome-wide linkage screen of bone mineral density (BMD) in European pedigrees ascertained through a male relative with low BMD values: evidence for quantitative trait loci on 17q21-23, 11q12-13, 13q12-14, and 22q11. J. Clin. Endocrinol. Metab. 93, 3755–3762 (2008).
Hsu, Y. H. et al. Variation in genes involved in the RANKL/RANK/OPG bone remodeling pathway are associated with bone mineral density at different skeletal sites in men. Hum. Genet. 118, 568–577 (2006).
Peacock, M. et al. Bone mineral density variation in men is influenced by sex-specific and non sex-specific quantitative trait loci. Bone 45, 443–448 (2009).
Xiao, P. et al. Genomic regions identified for BMD in a large sample including epistatic interactions and gender-specific effects. J. Bone Miner. Res. 21, 1536–1544 (2006).
Ioannidis, J. P. et al. Meta-analysis of genome-wide scans provides evidence for sex- and site-specific regulation of bone mass. J. Bone Miner. Res. 22, 173–183 (2007).
Ralston, S. H. & Uitterlinden, A. G. Genetics of osteoporosis. Endocr. Rev. 31, 629–662 (2010).
Richards, J. B. et al. Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann. Intern. Med. 151, 528–537 (2009).
Ferrari, S. L. et al. Polymorphisms in the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with variation in vertebral bone mass, vertebral bone size, and stature in whites. Am. J. Hum. Genet. 74, 866–875 (2004).
van Meurs, J. B. et al. Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA 299, 1277–1290 (2008).
Richards, J. B. et al. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 371, 1505–1512 (2008). Together with reference 21, these papers were the first GWASs for osteoporosis, and they made it clear that there were no common genetic variants of large effect for osteoporosis.
Styrkarsdottir, U. et al. Multiple genetic loci for bone mineral density and fractures. N. Engl. J. Med. 358, 2355–2365 (2008).
Styrkarsdottir, U. et al. New sequence variants associated with bone mineral density. Nature Genet. 41, 15–17 (2009).
Timpson, N. J. et al. Common variants in the region around Osterix are associated with bone mineral density and growth in childhood. Hum. Mol. Genet. 18, 1510–1517 (2009). This is the only GWAS for BMD that has been conducted in children.
Xiong, D. H. et al. Genome-wide association and follow-up replication studies identified ADAMTS18 and TGFBR3 as bone mass candidate genes in different ethnic groups. Am. J. Hum. Genet. 84, 388–398 (2009).
Rivadeneira, F. et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nature Genet. 41, 1199–1206 (2009). This is the first large-scale consortium-style GWAS for osteoporosis. This paper identified many clinically relevant drug targets for osteoporosis and substantially expanded the number of loci associated with BMD.
Estrada, K. et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nature Genet. 44, 491–501 (2012). This is the largest global effort to describe the genetic determinants of BMD and fracture. Using BMD loci, this paper was also the first to assess the contribution of BMD GWAS loci to fracture in a large sample size.
Morris, A. P. Transethnic meta-analysis of genomewide association studies. Genet. Epidemiol. 35, 809–822 (2011).
Dastani, Z. et al. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 8, e1002607 (2012).
Duncan, E. L. et al. Genome-wide association study using extreme truncate selection identifies novel genes affecting bone mineral density and fracture risk. PLoS Genet. 7, e1001372 (2011). This is an important paper demonstrating the relative use of selecting extremes of a phenotypic continuum to identify common genetic variants.
Hsu, Y. H. et al. An integration of genome-wide association study and gene expression profiling to prioritize the discovery of novel susceptibility loci for osteoporosis-related traits. PLoS Genet. 6, e1000977 (2010).
Zheng, H. et al. WNT16 influences bone mineral density, cortical bone thickness, bone strength and osteoporotic fracture risk. PLoS Genet. 5 Jul 2012 (doi: 10.1371/journal.pgen.1002745). Together with reference 32, this paper showed that forearm and total body BMD had allelic architectures that appeared to be less polygenic than lumbar spine and femoral neck sites.
Medina-Gomez, C. et al. Meta-analysis of genome-wide scans for total body BMD in children and adults reveals allelic heterogeneity and age-specific effects at the WNT16 locus. PLoS Genet. 5 Jul 2012 (doi: 10.1371/journal.pgen.1002718).
Frazer, K. A. et al. A second generation human haplotype map of over 3.1 million SNPs. Nature 449, 851–861 (2007).
Visscher, P. M., Hill, W. G. & Wray, N. R. Heritability in the genomics era—concepts and misconceptions. Nature Rev. Genet. 9, 255–266 (2008).
Guo, Y. et al. Genome-wide association study identifies ALDH7A1 as a novel susceptibility gene for osteoporosis. PLoS Genet. 6, e1000806 (2010).
Cummings, S. R. et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 361, 756–765 (2009).
Duncan, E. L. & Brown, M. A. Clinical review 2: genetic determinants of bone density and fracture risk—state of the art and future directions. J. Clin. Endocrinol. Metabolism 95, 2576–2587 (2010).
Angers, S. & Moon, R. T. Proximal events in Wnt signal transduction. Nature Rev. Mol. Cell Biol. 10, 468–477 (2009).
Kearns, A. E., Khosla, S. & Kostenuik, P. J. Receptor activator of nuclear factor κB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr. Rev. 29, 155–192 (2008).
Komori, T. Signaling networks in RUNX2-dependent bone development. J. Cell. Biochem. 112, 750–755 (2011).
Kubota, T., Michigami, T. & Ozono, K. Wnt signaling in bone metabolism. J. Bone Miner. Metabolism 27, 265–271 (2009).
Guo, J. et al. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell. Metabolism 11, 161–171 (2010).
Akiyama, H. et al. Interactions between Sox9 and β-catenin control chondrocyte differentiation. Genes Dev. 18, 1072–1087 (2004).
Gong, Y. et al. Osteoporosis-pseudoglioma syndrome, a disorder affecting skeletal strength and vision, is assigned to chromosome region 11q12-13. Am. J. Hum. Genet. 59, 146–151 (1996).
Van Wesenbeeck, L. et al. Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am. J. Hum. Genet. 72, 763–771 (2003).
Balemans, W. et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 10, 537–543 (2001).
Leupin, O. et al. Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J. Bone Miner. Res. 22, 1957–1967 (2007).
Estrach, S., Ambler, C. A., Lo Celso, C., Hozumi, K. & Watt, F. M. Jagged 1 is a β-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development 133, 4427–4438 (2006).
Li, L. et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nature Genet. 16, 243–251 (1997).
Engin, F. et al. Dimorphic effects of Notch signaling in bone homeostasis. Nature Med. 14, 299–305 (2008).
Xiong, J. et al. Matrix-embedded cells control osteoclast formation. Nature Med. 17, 1235–1241 (2011).
Fuller, K., Wong, B., Fox, S., Choi, Y. & Chambers, T. J. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J. Exp. Med. 188, 997–1001 (1998).
Simonet, W. S. et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89, 309–319 (1997).
Mizuno, A. et al. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem. Biophys. Res. Commun. 247, 610–615 (1998).
Li, Y. et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood 109, 3839–3848 (2007).
Farooqi, I. S. et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341, 879–884 (1999).
Sobacchi, C. et al. Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nature Genet. 39, 960–962 (2007).
Guerrini, M. M. et al. Human osteoclast-poor osteopetrosis with hypogammaglobulinemia due to TNFRSF11A (RANK) mutations. Am. J. Hum. Genet. 83, 64–76 (2008).
Whyte, M. P. et al. Osteoprotegerin deficiency and juvenile Paget's disease. N. Engl. J. Med. 347, 175–184 (2002).
Albagha, O. M. et al. Genome-wide association study identifies variants at CSF1, OPTN and TNFRSF11A as genetic risk factors for Paget's disease of bone. Nature Genet. 42, 520–524 (2010).
Karsenty, G., Kronenberg, H. M. & Settembre, C. Genetic control of bone formation. Annu. Rev. Cell Dev. Biol. 25, 629–648 (2009).
Amizuka, N. et al. Haploinsufficiency of parathyroid hormone-related peptide (PTHrP) results in abnormal postnatal bone development. Dev. Biol. 175, 166–176 (1996).
Smits, P., Dy, P., Mitra, S. & Lefebvre, V. Sox5 and Sox6 are needed to develop and maintain source, columnar, and hypertrophic chondrocytes in the cartilage growth plate. J. Cell Biol. 164, 747–758 (2004).
Nakashima, K. et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108, 17–29 (2002).
Lee, B. et al. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nature Genet. 16, 307–310 (1997).
Canalis, E. Update in new anabolic therapies for osteoporosis. J. Clin. Endocrinol. Metab. 95, 1496–1504 (2010).
Lewiecki, E. M. Sclerostin: a novel target for intervention in the treatment of osteoporosis. Discovery Med. 12, 263–273 (2011).
Gauthier, J. Y. et al. The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg. Med. Chem. Lett. 18, 923–928 (2008).
McClellan, J. & King, M. C. Genetic heterogeneity in human disease. Cell 141, 210–217 (2010).
Leslie, W. D. et al. Independent clinical validation of a Canadian FRAX tool: fracture prediction and model calibration. J. Bone Miner. Res. 25, 2350–2358 (2010).
Pepe, M. S., Gu, J. W. & Morris, D. E. The potential of genes and other markers to inform about risk. Cancer Epidemiol. Biomarkers Prev. 19, 655–665 (2010).
Yang, J. et al. Genome partitioning of genetic variation for complex traits using common SNPs. Nature Genet. 43, 519–525 (2011).
Ladouceur, M., Dastani, Z., Aulchenko, Y. S., Greenwood, C. M. & Richards, J. B. The empirical power of rare variant association methods: results from Sanger sequencing in 1,998 individuals. PLoS Genet. 8, e1002496 (2012).
Ladouceur, M., Leslie, W. D., Dastani, Z., Goltzman, D. & Richards, J. B. An efficient paradigm for genetic epidemiology cohort creation. PLoS ONE 5, e14045 (2010).
Bell, J. T. & Spector, T. D. A twin approach to unraveling epigenetics. Trends Genet. 27, 116–125 (2011).
Talens, R. P. et al. Variation, patterns, and temporal stability of DNA methylation: considerations for epigenetic epidemiology. FASEB J. 24, 3135–3144 (2010).
Suhre, K. et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 477, 54–60 (2011).
Kim, K. A. et al. R-spondin family members regulate the Wnt pathway by a common mechanism. Mol. Biol. Cell 19, 2588–2596 (2008).
Josse, R. G. Bone biology and the role of RANK/RANKL/OPG pathway. HealthPlexus [online], (2008).
Mackie, E. J., Tatarczuch, L. & Mirams, M. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J. Endocrinol. 211, 109–121 (2011).
Kung, A. W. et al. Association of JAG1 with bone mineral density and osteoporotic fractures: a genome-wide association study and follow-up replication studies. Am. J. Hum. Genet. 86, 229–239 (2010).
Koller, D. L. et al. Genome-wide association study of bone mineral density in premenopausal European-American women and replication in African-American women. J. Clin. Endocrinol. Metabolism 95, 1802–1809 (2010).
Ettinger, B. et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) investigators. JAMA 282, 637–645 (1999).
Neer, R. M. et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 344, 1434–1441 (2001).
Greenspan, S. L. et al. Effect of recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann. Internal Med. 146, 326–339 (2007).
Liberman, U. A. et al. Effect of oral alendronate on bone mineral. Density and the incidence of fractures in postmenopausal osteoporosis. New Engl. J. Med. 333, 1437–1444 (1995).
Rossouw, J. E. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 288, 321–333 (2002).
Acknowledgements
This work has been supported by the Canadian Institutes of Health Research, the Lady Davis Institute for Medical Research, Ministère de Développement économique, de l'Innovation et de l'Exportation du Québec, the Arthritis Research Campaign, the Wellcome Trust, Guy's & St. Thomas' NHS Foundation Trust and the King's College London Biomedical Centre. We would like to acknowledge the contributions of F. Rivadeneira, C. Greenwood and C. Polychronakos for their input on this Review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- deCODE genetics
-
An Icelandic company that specializes in the identification of genetic risk factors for disease.
- Osteoporosis-pseudoglioma syndrome
-
An autosomal recessive disorder conferring juvenile osteoporosis and juvenile-onset blindness that is caused by mutations in lipoprotein-receptor-related protein 5 (LRP5).
- Osteopetrosis
-
A syndrome of high bone mass caused by an imbalance in the constant remodelling of bone, which favours bone formation, or mutations leading to increased bone formation, such as activating mutations in low-density lipoprotein receptor-related protein 5 (LRP5).
- Paget's disease
-
Focalized bone lesions characterized by enhanced bone remodelling and resultant overgrowth of bone, leading to an increased risk of fracture.
- Cleidocranial dysplasia
-
An autosomal dominant condition characterized by defective differentiation of osteoblasts, resulting in impaired bone formation, short stature and abnormal teeth owing to mutations of runt-related transcription factor 2 (RUNX2), which encodes core-binding factor alpha 1.
Rights and permissions
About this article
Cite this article
Richards, J., Zheng, HF. & Spector, T. Genetics of osteoporosis from genome-wide association studies: advances and challenges. Nat Rev Genet 13, 576–588 (2012). https://doi.org/10.1038/nrg3228
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg3228
This article is cited by
-
A new hip fracture risk index derived from FEA-computed proximal femur fracture loads and energies-to-failure
Osteoporosis International (2024)
-
Association between AXIN1 gene polymorphism (rs9921222) of WNT signaling pathway and susceptibility to osteoporosis in Egyptian patients: a case-control study
BMC Musculoskeletal Disorders (2023)
-
Genome-wide polygenic risk score for major osteoporotic fractures in postmenopausal women using associated single nucleotide polymorphisms
Journal of Translational Medicine (2023)
-
Causal influence of muscle weakness on cardiometabolic diseases and osteoporosis
Scientific Reports (2023)
-
Heel bone mineral density and various oral diseases: a bidirectional Mendelian randomization
Journal of Bone and Mineral Metabolism (2023)