Key Points
-
Experimental approaches such as genetic screening, mRNA expression profiling, and Argonaute crosslinking and immunoprecipitation aim to identify microRNA (miRNA) targets and/or individual binding sites within them. Computational analyses of the resultant high-throughput data reveal the miRNA targets with high accuracy and resolution.
-
Factors that define functional miRNA interaction sites include miRNA 'seed' complementarity, structural accessibility, and sequence and positional biases. These factors support modulatory interactions with RNA-binding proteins.
-
miRNA seed families and clusters of co-expressed miRNAs are prevalent and may contribute to regulation of individual pathways across tissues and developmental stages.
-
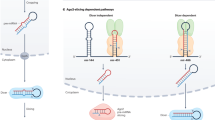
'Non-canonical' miRNA-binding sites seem to be prevalent, and their functionality should be further investigated.
-
Outcomes of miRNA–target interactions include repression and increased precision of target gene expression, as well as induction of correlations in the expression levels of different targets.
-
Computational modelling of miRNA–target interactions has provided insights into their consequences on target expression.
Abstract
Comparative genomics analyses and high-throughput experimental studies indicate that a microRNA (miRNA) binds to hundreds of sites across the transcriptome. Although the knockout of components of the miRNA biogenesis pathway has profound phenotypic consequences, most predicted miRNA targets undergo small changes at the mRNA and protein levels when the expression of the miRNA is perturbed. Alternatively, miRNAs can establish thresholds in and increase the coherence of the expression of their target genes, as well as reduce the cell-to-cell variability in target gene expression. Here, we review the recent progress in identifying miRNA targets and the emerging paradigms of how miRNAs shape the dynamics of target gene expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
16 September 2014
In Box 3 of this article, equation 3 was given incorrectly. The article has been corrected online. The authors apologize for this error.
References
Wightman, B., Ha, I. & Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75, 855–862 (1993).
Lee, R. C., Feinbaum, R. L. & Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843–854 (1993).
Pasquinelli, A. E. et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 408, 86–89 (2000).
Reinhart, B. J. et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403, 901–906 (2000).
Huntzinger, E. & Izaurralde, E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nature Rev. Genet. 12, 99–110 (2011).
Ha, I., Wightman, B. & Ruvkun, G. A bulged lin-4/lin-14 RNA duplex is sufficient for Caenorhabditis elegans lin-14 temporal gradient formation. Genes Dev. 10, 3041–3050 (1996).
Brennecke, J., Stark, A., Russell, R. B. & Cohen, S. M. Principles of microRNA–target recognition. PLoS Biol. 3, e85 (2005).
Grimson, A. et al. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol. Cell 27, 91–105 (2007).
Khorshid, M., Hausser, J., Zavolan, M. & van Nimwegen, E. A biophysical miRNA–mRNA interaction model infers canonical and noncanonical targets. Nature Methods 10, 253–255 (2013).
Lewis, B. P., Shih, I. H., Jones-Rhoades, M. W., Bartel, D. P. & Burge, C. B. Prediction of mammalian microRNA targets. Cell 115, 787–798 (2003).
Lewis, B. P., Burge, C. B. & Bartel, D. P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20 (2005).
Lim, L. P. et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433, 769–773 (2005).
Gaidatzis, D., van Nimwegen, E., Hausser, J. & Zavolan, M. Inference of miRNA targets using evolutionary conservation and pathway analysis. BMC Bioinformatics 8, 69 (2007).
Krek, A. et al. Combinatorial microRNA target predictions. Nature Genet. 37, 495–500 (2005).
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Friedman, R. C., Farh, K. K., Burge, C. B. & Bartel, D. P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19, 92–105 (2009).
Kozomara, A. & Griffiths-Jones, S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 42, D68–D73 (2014).
Chi, S. W., Zang, J. B., Mele, A. & Darnell, R. B. Argonaute HITS-CLIP decodes microRNA–mRNA interaction maps. Nature 460, 479–486 (2009).
Hafner, M. et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 141, 129–141 (2010).
Chi, S. W., Hannon, G. J. & Darnell, R. B. An alternative mode of microRNA target recognition. Nature Struct. Mol. Biol. 19, 321–327 (2012). This study identifies a prevalent type of non-canonical miRNA-binding sites — namely, the pivot sites.
Loeb, G. B. et al. Transcriptome-wide miR-155 binding map reveals widespread noncanonical microRNA targeting. Mol. Cell 48, 760–770 (2012).
Helwak, A., Kudla, G., Dudnakova, T. & Tollervey, D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 153, 654–665 (2013).
Betel, D., Koppal, A., Agius, P., Sander, C. & Leslie, C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 11, R90 (2010).
Kanellopoulou, C. et al. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 19, 489–501 (2005).
Yi, R., Poy, M. N., Stoffel, M. & Fuchs, E. A skin microRNA promotes differentiation by repressing 'stemness'. Nature 452, 225–229 (2008).
Tay, Y., Zhang, J., Thomson, A. M., Lim, B. & Rigoutsos, I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature 455, 1124–1128 (2008).
Cheung, T. H. et al. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature 482, 524–528 (2012).
Muljo, S. A. et al. Aberrant T cell differentiation in the absence of Dicer. J. Exp. Med. 202, 261–269 (2005).
Koralov, S. B. et al. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell 132, 860–874 (2008).
Chen, J. F. et al. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc. Natl Acad. Sci. USA 105, 2111–2116 (2008).
Poy, M. N. et al. miR-375 maintains normal pancreatic α- and β-cell mass. Proc. Natl Acad. Sci. USA 106, 5813–5818 (2009).
Levine, E., Zhang, Z., Kuhlman, T. & Hwa, T. Quantitative characteristics of gene regulation by small RNA. PLoS Biol. 5, e229 (2007). This paper introduces the threshold-linear response of small RNA targets to their transcriptional induction.
Mukherji, S. et al. MicroRNAs can generate thresholds in target gene expression. Nature Genet. 43, 854–859 (2011). This paper shows the threshold-linear response with a miRNA target reporter construct in mammalian cells.
Osella, M., Bosia, C., Cora, D. & Caselle, M. The role of incoherent microRNA-mediated feedforward loops in noise buffering. PLoS Comput. Biol. 7, e1001101 (2011). This study computationally explores the consequences of a gene being regulated only at the transcriptional level or by both a transcription factor and a miRNA that collectively form a FFL.
Poliseno, L. et al. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465, 1033–1038 (2010).
Islam, S. et al. Characterization of the single-cell transcriptional landscape by highly multiplex RNA-seq. Genome Res. 21, 1160–1167 (2011).
Wee, L. M., Flores-Jasso, C. F., Salomon, W. E. & Zamore, P. D. Argonaute divides its RNA guide into domains with distinct functions and RNA-binding properties. Cell 151, 1055–1067 (2012). This study assesses the importance of different miRNA positions for the interaction with an RNA target in the context of miRISC and provides a substantial number of measurements of rate constants of miRNA–target interaction.
Pasquinelli, A. E. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nature Rev. Genet. 13, 271–282 (2012).
Thomson, D. W., Bracken, C. P. & Goodall, G. J. Experimental strategies for microRNA target identification. Nucleic Acids Res. 39, 6845–6853 (2011).
Hipfner, D. R., Weigmann, K. & Cohen, S. M. The bantam gene regulates Drosophila growth. Genetics 161, 1527–1537 (2002).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Wang, T., Wei, J. J., Sabatini, D. M. & Lander, E. S. Genetic screens in human cells using the CRISPR–Cas9 system. Science 343, 80–84 (2014).
Miska, E. A. et al. Most Caenorhabditis elegans microRNAs are individually not essential for development or viability. PLoS Genet. 3, e215 (2007).
Park, C. Y. et al. A resource for the conditional ablation of microRNAs in the mouse. Cell Rep. 1, 385–391 (2012).
Linsley, P. S. et al. Transcripts targeted by the microRNA-16 family cooperatively regulate cell cycle progression. Mol. Cell. Biol. 27, 2240–2252 (2007).
Selbach, M. et al. Widespread changes in protein synthesis induced by microRNAs. Nature 455, 58–63 (2008).
Baek, D. et al. The impact of microRNAs on protein output. Nature 455, 64–71 (2008).
Guo, H., Ingolia, N. T., Weissman, J. S. & Bartel, D. P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466, 835–840 (2010).
Hausser, J. et al. Timescales and bottlenecks in miRNA-dependent gene regulation. Mol. Syst. Biol. 9, 711 (2013). This study integrates a range of data sets to estimate kinetic parameters for miRNA-dependent target regulation.
Karginov, F. V. et al. A biochemical approach to identifying microRNA targets. Proc. Natl Acad. Sci. USA 104, 19291–19296 (2007).
Zhang, L. et al. Systematic identification of C. elegans miRISC proteins, miRNAs, and mRNA targets by their interactions with GW182 proteins AIN-1 and AIN-2. Mol. Cell 28, 598–613 (2007).
Meier, J. et al. Genome-wide identification of translationally inhibited and degraded miR-155 targets using RNA-interacting protein-IP. RNA Biol. 10, 1018–1029 (2013).
Zisoulis, D. G. et al. Comprehensive discovery of endogenous Argonaute binding sites in Caenorhabditis elegans. Nature Struct. Mol. Biol. 17, 173–179 (2010).
Kishore, S. et al. A quantitative analysis of CLIP methods for identifying binding sites of RNA-binding proteins. Nature Methods 8, 559–564 (2011).
Hausser, J., Syed, A. P., Bilen, B. & Zavolan, M. Analysis of CDS-located miRNA target sites suggests that they can effectively inhibit translation. Genome Res. 23, 604–615 (2013).
Zhang, C. & Darnell, R. B. Mapping in vivo protein-RNA interactions at single-nucleotide resolution from HITS-CLIP data. Nature Biotech. 29, 607–614 (2011).
Konig, J. et al. iCLIP — transcriptome-wide mapping of protein–RNA interactions with individual nucleotide resolution. J. Vis. Exp. 50, e2638 (2011).
Broughton, J. P. & Pasquinelli, A. E. Identifying Argonaute binding sites in Caenorhabditis elegans using iCLIP. Methods 63, 119–125 (2013).
Smith, K. C. Photochemical addition of amino acids to 14C-uracil. Biochem. Biophys. Res. Commun. 34, 354–357 (1969).
Shetlar, M. D., Carbone, J., Steady, E. & Hom, K. Photochemical addition of amino acids and peptides to polyuridylic acid. Photochem. Photobiol. 39, 141–144 (1984).
Hockensmith, J. W., Kubasek, W. L., Vorachek, W. R. & von Hippel, P. H. Laser cross-linking of nucleic acids to proteins. Methodology and first applications to the phage T4 DNA replication system. J. Biol. Chem. 261, 3512–3518 (1986).
Lai, E. C. MicroRNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nature Genet. 30, 363–364 (2002).
Wang, Y., Sheng, G., Juranek, S., Tuschl, T. & Patel, D. J. Structure of the guide-strand-containing argonaute silencing complex. Nature 456, 209–213 (2008).
Wang, Y. et al. Nucleation, propagation and cleavage of target RNAs in Ago silencing complexes. Nature 461, 754–761 (2009).
Parker, J. S., Parizotto, E. A., Wang, M., Roe, S. M. & Barford, D. Enhancement of the seed–target recognition step in RNA silencing by a PIWI/MID domain protein. Mol. Cell 33, 204–214 (2009). This paper evaluates the influence of a PIWI/MID domain on the strength of interaction between a small RNA and a target, which reveals that the organization of the small RNA seed sequence by AGO reduces the entropy penalty for the interaction with the target.
Doench, J. G. & Sharp, P. A. Specificity of microRNA target selection in translational repression. Genes Dev. 18, 504–511 (2004).
Yekta, S., Shih, I. H. & Bartel, D. P. MicroRNA-directed cleavage of HOXB8 mRNA. Science 304, 594–596 (2004).
Alexiou, P., Maragkakis, M., Papadopoulos, G. L., Reczko, M. & Hatzigeorgiou, A. G. Lost in translation: an assessment and perspective for computational microRNA target identification. Bioinformatics 25, 3049–3055 (2009).
Hausser, J., Landthaler, M., Jaskiewicz, L., Gaidatzis, D. & Zavolan, M. Relative contribution of sequence and structure features to the mRNA binding of Argonaute/EIF2C–miRNA complexes and the degradation of miRNA targets. Genome Res. 19, 2009–2020 (2009).
Ebert, M. S. & Sharp, P. A. Roles for microRNAs in conferring robustness to biological processes. Cell 149, 515–524 (2012).
Jackson, A. L. et al. Widespread siRNA “off-target” transcript silencing mediated by seed region sequence complementarity. RNA 12, 1179–1187 (2006).
Kim, Y. K. et al. Functional links between clustered microRNAs: suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res. 37, 1672–1681 (2009).
Kozomara, A. & Griffiths-Jones, S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 39, D152–D157 (2011).
Benetti, R. et al. A mammalian microRNA cluster controls DNA methylation and telomere recombination via Rbl2-dependent regulation of DNA methyltransferases. Nature Struct. Mol. Biol. 15, 268–279 (2008).
Sinkkonen, L. et al. MicroRNAs control de novo DNA methylation through regulation of transcriptional repressors in mouse embryonic stem cells. Nature Struct. Mol. Biol. 15, 259–267 (2008).
Wang, Q. et al. miR-17–92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc. Natl Acad. Sci. USA 105, 2889–2894 (2008).
Houbaviy, H. B., Dennis, L., Jaenisch, R. & Sharp, P. A. Characterization of a highly variable eutherian microRNA gene. RNA 11, 1245–1257 (2005).
Houbaviy, H. B., Murray, M. F. & Sharp, P. A. Embryonic stem cell-specific microRNAs. Dev. Cell 5, 351–358 (2003).
Chen, P. Y. et al. The developmental miRNA profiles of zebrafish as determined by small RNA cloning. Genes Dev. 19, 1288–1293 (2005).
Giraldez, A. J. et al. MicroRNAs regulate brain morphogenesis in zebrafish. Science 308, 833–838 (2005).
Tang, G. Q. & Maxwell, E. S. Xenopus microRNA genes are predominantly located within introns and are differentially expressed in adult frog tissues via post-transcriptional regulation. Genome Res. 18, 104–112 (2008).
Altuvia, Y. et al. Clustering and conservation patterns of human microRNAs. Nucleic Acids Res. 33, 2697–2706 (2005).
Aravin, A. A. et al. The small RNA profile during Drosophila melanogaster development. Dev. Cell 5, 337–350 (2003).
Landgraf, P. et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 129, 1401–1414 (2007).
Suh, M. R. et al. Human embryonic stem cells express a unique set of microRNAs. Dev. Biol. 270, 488–498 (2004).
Bentwich, I. et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nature Genet. 37, 766–770 (2005).
Mohammed, J., Flynt, A. S., Siepel, A. & Lai, E. C. The impact of age, biogenesis, and genomic clustering on Drosophila microRNA evolution. RNA 19, 1295–1308 (2013).
Lal, A. et al. miR-24 Inhibits cell proliferation by targeting E2F2, MYC, and other cell-cycle genes via binding to “seedless” 3′UTR microRNA recognition elements. Mol. Cell 35, 610–625 (2009).
Shin, C. et al. Expanding the microRNA targeting code: functional sites with centered pairing. Mol. Cell 38, 789–802 (2010).
Majoros, W. H. & Ohler, U. Spatial preferences of microRNA targets in 3′ untranslated regions. BMC Genomics 8, 152 (2007).
Meijer, H. A. et al. Translational repression and eIF4A2 activity are critical for microRNA-mediated gene regulation. Science 340, 82–85 (2013).
Gu, S., Jin, L., Zhang, F., Sarnow, P. & Kay, M. A. Biological basis for restriction of microRNA targets to the 3′ untranslated region in mammalian mRNAs. Nature Struct. Mol. Biol. 16, 144–150 (2009).
Fang, Z. & Rajewsky, N. The impact of miRNA target sites in coding sequences and in 3′UTRs. PLoS ONE 6, e18067 (2011).
Reczko, M., Maragkakis, M., Alexiou, P., Grosse, I. & Hatzigeorgiou, A. G. Functional microRNA targets in protein coding sequences. Bioinformatics 28, 771–776 (2012).
Kertesz, M., Iovino, N., Unnerstall, U., Gaul, U. & Segal, E. The role of site accessibility in microRNA target recognition. Nature Genet. 39, 1278–1284 (2007).
Tafer, H. et al. The impact of target site accessibility on the design of effective siRNAs. Nature Biotech. 26, 578–583 (2008).
Nielsen, C. B. et al. Determinants of targeting by endogenous and exogenous microRNAs and siRNAs. RNA 13, 1894–1910 (2007).
Jacobsen, A., Wen, J., Marks, D. S. & Krogh, A. Signatures of RNA binding proteins globally coupled to effective microRNA target sites. Genome Res. 20, 1010–1019 (2010).
Kedde, M. et al. RNA-binding protein Dnd1 inhibits microRNA access to target mRNA. Cell 131, 1273–1286 (2007).
Bhattacharyya, S. N., Habermacher, R., Martine, U., Closs, E. I. & Filipowicz, W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell 125, 1111–1124 (2006).
Kedde, M. et al. A Pumilio-induced RNA structure switch in p27-3′ UTR controls miR-221 and miR-222 accessibility. Nature Cell Biol. 12, 1014–1020 (2010).
Li, J. et al. Identifying mRNA sequence elements for target recognition by human Argonaute proteins. Genome Res. 24, 775–785 (2014).
Vlachos, I. S. & Hatzigeorgiou, A. G. Online resources for miRNA analysis. Clin. Biochem. 46, 879–900 (2013).
Song, J. L. et al. Select microRNAs are essential for early development in the sea urchin. Dev. Biol. 362, 104–113 (2012).
Ohrt, T. et al. Fluorescence correlation spectroscopy and fluorescence cross-correlation spectroscopy reveal the cytoplasmic origination of loaded nuclear RISC in vivo in human cells. Nucleic Acids Res. 36, 6439–6449 (2008). The data obtained in this study enable the estimation of the rates of AGO loading and unloading with small RNAs.
Schwanhausser, B. et al. Global quantification of mammalian gene expression control. Nature 473, 337–342 (2011).
Nam, J. W. et al. Global analyses of the effect of different cellular contexts on microRNA targeting. Mol. Cell 53, 1031–1043 (2014).
Enright, A. J. et al. MicroRNA targets in Drosophila. Genome Biol. 5, R1 (2003).
Shalgi, R., Lieber, D., Oren, M. & Pilpel, Y. Global and local architecture of the mammalian microRNA–transcription factor regulatory network. PLoS Comput. Biol. 3, e131 (2007).
Hornstein, E. & Shomron, N. Canalization of development by microRNAs. Nature Genet 38, S20–S24 (2006).
Marson, A. et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell 134, 521–533 (2008).
Re, A., Cora, D., Taverna, D. & Caselle, M. Genome-wide survey of microRNA–transcription factor feed-forward regulatory circuits in human. Mol. Biosyst 5, 854–867 (2009).
Arvey, A., Larsson, E., Sander, C., Leslie, C. S. & Marks, D. S. Target mRNA abundance dilutes microRNA and siRNA activity. Mol. Syst. Biol. 6, 363 (2010).
Ebert, M. S., Neilson, J. R. & Sharp, P. A. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nature Methods 4, 721–726 (2007).
Cazalla, D., Yario, T. & Steitz, J. A. Down-regulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. Science 328, 1563–1566 (2010).
Marcinowski, L. et al. Degradation of cellular miR-27 by a novel, highly abundant viral transcript is important for efficient virus replication in vivo. PLoS Pathog. 8, e1002510 (2012).
Wang, Y. et al. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev. Cell 25, 69–80 (2013). This is a study of crosstalk between a lncRNA and mRNA targets of miRNAs, in which the abundance of the involved molecular species have been measured.
Kallen, A. N. et al. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol. Cell 52, 101–112 (2013).
Chang, J. et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 1, 106–113 (2004).
Denzler, R., Agarwal, V., Stefano, J., Bartel, D. P. & Stoffel, M. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol. Cell 54, 766–776 (2014).
Hansen, T. B. et al. Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388 (2013).
Memczak, S. et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338 (2013).
Bosia, C., Pagnani, A. & Zecchina, R. Modelling competing endogenous RNA networks. PLoS ONE 8, e66609 (2013).
Figliuzzi, M., Marinari, E. & De Martino, A. MicroRNAs as a selective channel of communication between competing RNAs: a steady-state theory. Biophys. J. 104, 1203–1213 (2013).
Buchler, N. E. & Louis, M. Molecular titration and ultrasensitivity in regulatory networks. J. Mol. Biol. 384, 1106–1119 (2008).
Larsson, E., Sander, C. & Marks, D. mRNA turnover rate limits siRNA and microRNA efficacy. Mol. Syst. Biol. 6, 433 (2010).
Kim, H. H. et al. HuR recruits let-7/RISC to repress c-Myc expression. Genes Dev. 23, 1743–1748 (2009).
Erhard, F. et al. Widespread context-dependency of microRNA-mediated regulation. Genome Res. 24, 906–919 (2014).
Majoros, W. H. et al. MicroRNA target site identification by integrating sequence and binding information. Nature Methods 10, 630–633 (2013).
Erhard, F., Dolken, L., Jaskiewicz, L. & Zimmer, R. PARma: identification of microRNA target sites in AGO-PAR-CLIP data. Genome Biol. 14, R79 (2013).
Grun, D., Wang, Y. L., Langenberger, D. & Gunsalus, K. C. & Rajewsky, N. microRNA target predictions across seven Drosophila species and comparison to mammalian targets. PLoS Comput. Biol. 1, e13 (2005).
He, L. et al. A microRNA polycistron as a potential human oncogene. Nature 435, 828–833 (2005).
Franceschini, A. et al. STRING v9.1: protein–protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 41, D808–D815 (2013).
Wang, D. et al. Quantitative functions of Argonaute proteins in mammalian development. Genes Dev. 26, 693–704 (2012).
Stalder, L. et al. The rough endoplasmatic reticulum is a central nucleation site of siRNA-mediated RNA silencing. EMBO J. 32, 1115–1127 (2013).
Djuranovic, S., Nahvi, A. & Green, R. miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science 336, 237–240 (2012).
Bethune, J., Artus-Revel, C. G. & Filipowicz, W. Kinetic analysis reveals successive steps leading to miRNA-mediated silencing in mammalian cells. EMBO Rep. 13, 716–723 (2012).
Bazzini, A. A., Lee, M. T. & Giraldez, A. J. Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science 336, 233–237 (2012).
Acknowledgements
The authors thank P. Pemberton-Ross for help with the figure in Box 2 and members of M.Z.'s laboratory for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Argonaute
-
(AGO). A protein that, together with a microRNA (miRNA), forms a minimal miRNA-induced silencing complex (miRISC). Although the number of AGO proteins varies across species, four paralogues (which are thought to have overlapping activities) are known in humans and mice.
- miRNA-induced silencing complex
-
(miRISC). A ribonucleoprotein complex that includes Argonaute (AGO), a microRNA (miRNA) and additional proteins such as Dicer and trinucleotide repeat-containing gene 6A protein (TNRC6A). The miRNA in this complex guides the AGO protein to targets.
- CRISPR–Cas
-
(Clustered regularly interspaced short palindromic repeat–CRISPR-associated). A system that mediates the RNA-based immune defence of bacteria and archea to viruses and plasmids. It is composed of a genomic CRISPR array (which contains virus- or plasmid- complementary sequences that are interspersed with repeat elements) and a Cas protein (which carries the endonuclease activity).
- Stable isotope labelling by amino acids in cell culture
-
(SILAC). A mass spectrometry-based experimental technique that is used to compare protein abundance in different experimental conditions. Cells of one sample are grown in a medium containing amino acids labelled with light isotopes, and cells of another sample are grown in medium with heavy-isotope-labelled amino acids. The samples are then mixed, and changes in protein abundance are determined from the ratio between the signal from the light and heavy isotopes.
- Ribosome profiling
-
An experimental method to quantify the translation efficiency of individual mRNAs. Sucrose gradients are used to separate mRNAs that are actively translated and that are therefore associated with multiple ribosomes. Quantification can be done either at the level of the whole transcript (as in polysome profiling) or of the precise regions in the mRNA that are bound by ribosomes, which are protected from nuclease digestion (as in ribosome profiling or footprinting).
- Crosslinking and immunoprecipitation
-
(CLIP). An experimental method to map the binding sites of RNA-binding proteins across the transcriptome. Proteins are crosslinked to RNA using ultraviolet light, and an antibody is used to specifically isolate the RNA-binding protein of interest together with its RNA interaction partners, which are subjected to sequencing.
- Photoactivatable ribonucleoside-enhanced CLIP
-
(PAR-CLIP). A variant of CLIP in which photoreactive ribonucleoside analogues such as 4-thiouridine are incorporated into RNAs before crosslinking with ultraviolet A light (the wavelength of which is 365 nm).
- High-throughput sequencing of RNAs isolated by CLIP
-
(HITS-CLIP). A variant of CLIP in which the crosslinking is achieved using ultraviolet C light (the wavelength of which is 254 nm).
- Individual-nucleotide resolution CLIP
-
(iCLIP). A variant of CLIP that, in contrast to the other methods that rely on the reverse transcriptase to polymerize beyond the crosslinked nucleotides, aims to sequence the cDNAs at which the reverse transcriptase stopped at the crosslinked nucleotides, thereby achieving individual-nucleotide resolution in the mapping of sites of RNA-binding proteins.
- Crosslinking, immunoprecipitation and sequencing of hybrids
-
(CLASH). An experimental method to isolate RNAs that interact by hybridization in a ribonucleoprotein complex. The complex is immunoprecipitated with a specific antibody, the RNA is partially fragmented, interacting RNAs are ligated and the resulting chimeric products are sequenced.
- Long non-coding RNA
-
(lncRNA). An RNA molecule that is generally longer than structural RNAs (such as tRNAs, small nuclear RNAs and small nucleolar RNAs) and that does not encode proteins. Further subcategories are distinguished depending on the type of genomic regions from which they derive. One example is the long intergenic non-coding RNAs (lincRNAs) that are transcribed from genomic regions between protein-coding gene loci.
- Circular RNAs
-
(circRNAs). Very stable RNAs with circular structures that result from the ligation of the 3′ ends to the 5′ ends, for example, of exons. The circRNA CDR1 antisense RNA (CDR1as) has recently been found to function as a miRNA 'sponge'.
Rights and permissions
About this article
Cite this article
Hausser, J., Zavolan, M. Identification and consequences of miRNA–target interactions — beyond repression of gene expression. Nat Rev Genet 15, 599–612 (2014). https://doi.org/10.1038/nrg3765
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg3765
This article is cited by
-
The Impact of microRNA SNPs on Breast Cancer: Potential Biomarkers for Disease Detection
Molecular Biotechnology (2024)
-
Differential functions of RhoGDIβ in malignant transformation and progression of urothelial cell following N-butyl-N-(4-hydmoxybutyl) nitrosamine exposure
BMC Biology (2023)
-
Mesenchymal stem cells under epigenetic control – the role of epigenetic machinery in fate decision and functional properties
Cell Death & Disease (2023)
-
A review on immunomodulatory effects of BPA analogues
Archives of Toxicology (2023)
-
miR-212-5p Suppresses Glioma Development via Targeting SUMO2
Biochemical Genetics (2023)