Abstract
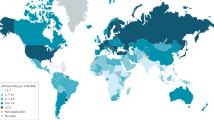
Pancreatic cancer, although infrequent, has an exceptionally high mortality rate, making it one of the four or five most common causes of cancer mortality in developed countries. The incidence of pancreatic cancer varies greatly across regions, which suggests roles for lifestyle factors, such as diet, or environmental factors, such as vitamin D exposure. Smoking is the most common known risk factor, and is the cause of 20–25% of all pancreatic tumors. Alcohol does not seem to be a risk factor, unless it leads to chronic pancreatitis, which is a probable risk factor. Long-standing diabetes increases the risk of pancreatic cancer, but can also be an early manifestation of pancreatic tumors. 5–10% of patients with pancreatic cancer have an underlying germline disorder, while the remaining percentage of cancer cases is thought to be caused by somatic mutations. Some individual studies suggest that mutations in various polymorphic genes can lead to small increases in the risk of pancreatic cancer, but these findings need to be replicated. Rising prevalence of smoking in developing countries, improved diagnosis and increasing population longevity are all likely to increase the global burden of pancreatic cancer in the coming decades.
Key Points
-
Despite its low incidence in developed countries, pancreatic cancer is associated with poor survival and ranks as the fourth or fifth most common cause of cancer mortality
-
Smoking causes 20–25% of pancreatic cancer cases and is the most frequent cause of this tumor, yet it is the most preventable
-
Chronic pancreatitis and diabetes are two benign diseases that have been linked to pancreatic cancer
-
5–10% of patients with pancreatic cancer have an underlying germline disorder, while the remaining cases seem to result from damage to genes occurred during life
-
Some apparently sporadic pancreatic cancers may in fact be caused by the interaction of polymorphic genes with other genes or with environmental factors
-
Potentially important risk factors that need further study include Helicobacter pylori and viral infections, and drugs such as aspirin, NSAIDS, and statins
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Parkin, D. M., Bray, F., Ferlay, J. & Pisani, P. Estimating the world cancer burden: Globocan 2000. Int. J. Cancer 94, 153–156 (2001).
Carpelan-Holmstrom, M. et al. Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the Finnish Cancer Registry. Gut 54, 385–387 (2005).
Curado, M. P. et al. (eds) Cancer Incidence in Five Continents Vol. 9 (IARC Scientific Publications No. 160, Lyon, 2007).
Kinoshita, S., Wagatsuma, Y. & Okada, M. Geographical distribution for malignant neoplasm of the pancreas in relation to selected climatic factors in Japan. Int. J. Health Geogr. 6, 34 (2007).
Giovannucci, E. et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Natl Cancer Inst. 98, 451–459 (2006).
Grant, W. B. & Mohr, S. B. Ecological studies of ultraviolet B, vitamin D and cancer since 2000. Ann. Epidemiol. 19, 446–454 (2009).
Parkin, D. M., Bray, F., Ferlay, J. & Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 55, 74–108 (2005).
Raimondi, S., Maisonneuve, P., Lohr, J. M. & Lowenfels, A. B. Early onset pancreatic cancer: evidence of a major role for smoking and genetic factors. Cancer Epidemiol. Biomarkers Prev. 16, 1894–1897 (2007).
Deutsch, M., Rosenstein, M. M. & Ramanathan, R. K. Pancreatic cancer in a young adult after treatment for Hodgkin's disease. Clin. Oncol. (R. Coll. Radiol.) 11, 280–282 (1999).
Wahi, M. M., Shah, N., Schrock, C. E., Rosemurgy, A. S. & Goldin, S. B. Reproductive factors and risk of pancreatic cancer in women: a review of the literature. Ann. Epidemiol. 19, 103–111 (2009).
Horner, M. J. et al. (Eds) SEER Cancer Statistics Review, 1975–2006, National Cancer Institute [online], (2009).
Silverman, D. T. et al. Why do Black Americans have a higher risk of pancreatic cancer than White Americans? Epidemiology 14, 45–54 (2003).
Ginde, A. A., Liu, M. C. & Camargo, C. A. J. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch. Intern. Med. 169, 626–632 (2009).
Muscat, J. E., Djordjevic, M. V., Colosimo, S., Stellman S. D. & Richie, J. P. J. Racial differences in exposure and glucuronidation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Cancer 103, 1420–1426 (2005).
Lichtenstein, P. et al. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 343, 78–85 (2000).
Jiao, L. et al. A combined healthy lifestyle score and risk of pancreatic cancer in a large cohort study. Arch. Intern. Med. 169, 764–770 (2009).
Iodice, S., Gandini, S., Maisonneuve, P. & Lowenfels, A. B. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch. Surg. 393, 535–545 (2008).
Boffetta, P., Hecht, S., Gray, N., Gupta, P. & Straif, K. Smokeless tobacco and cancer. Lancet Oncol. 9, 667–675 (2008).
Rohrmann, S. et al. Ethanol intake and the risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Causes Control 20, 785–794 (2009).
Jiao, L. et al. Alcohol use and risk of pancreatic cancer: the NIH-AARP Diet and Health Study. Am. J. Epidemiol. 169, 1043–1051 (2009).
Genkinger, J. M. et al. Alcohol intake and pancreatic cancer risk: a pooled analysis of fourteen cohort studies. Cancer Epidemiol. Biomarkers Prev. 18, 765–776 (2009).
Vrieling, A. et al. Fruit and vegetable consumption and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 124, 1926–1934 (2009).
Nothlings, U., Murphy, S. P., Wilkens, L. R., Henderson, B. E. & Kolonel, L. N. Flavonols and pancreatic cancer risk: the multiethnic cohort study. Am. J. Epidemiol. 166, 924–931 (2007).
Stolzenberg-Solomon, R. Z. et al. Dietary and other methyl-group availability factors and pancreatic cancer risk in a cohort of male smokers. Am. J. Epidemiol. 153, 680–687 (2001).
Larsson, S. C., Hakansson, N., Giovannucci, E. & Wolk, A. Folate intake and pancreatic cancer incidence: a prospective study of Swedish women and men. J. Natl Cancer Inst. 98, 407–413 (2006).
Giovannucci, E. Vitamin D and cancer incidence in the Harvard cohorts. Ann. Epidemiol. 19, 84–88 (2009).
Gong, Z., Holly, E. A. & Bracci, P. M. Intake of folate, vitamins B(6), B (12) and methionine and risk of pancreatic cancer in a large population-based case-control study. Cancer Causes Control doi: 10.1007/s10552-009-9352–9359
Larsson, S. C., Giovannucci, E. & Wolk, A. Methionine and vitamin B6 intake and risk of pancreatic cancer: a prospective study of Swedish women and men. Gastroenterology 132, 113–118 (2007).
Reeves, G. K. et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 335, 1134 (2007).
Fryzek, J. P., Schenk, M., Kinnard, M., Greenson, J. K. & Garabrant, D. H. The association of body mass index and pancreatic cancer in residents of southeastern Michigan, 1996–1999. Am. J. Epidemiol. 162, 222–228 (2005).
Patel, A. V. et al. Obesity, recreational physical activity, and risk of pancreatic cancer in a large, U. S. Cohort. Cancer Epidemiol. Biomarkers Prev. 14, 459–466 (2005).
Berrington de González, A., Sweetland, S. & Spencer, E. A meta-analysis of obesity and the risk of pancreatic cancer. Br. J. Cancer 89, 519–523 (2003).
Khasawneh, J. et al. Inflammation and mitochondrial fatty acid beta-oxidation link obesity to early tumor promotion. Proc. Natl Acad. Sci. USA 106, 3354–3359 (2009).
Jiao, L. et al. Glycemic index, carbohydrates, glycemic load, and the risk of pancreatic cancer in a prospective cohort study. Cancer Epidemiol. Biomarkers Prev. 18, 1144–1151 (2009).
Bao, Y. et al. Added sugar and sugar-sweetened foods and beverages and the risk of pancreatic cancer in the National Institutes of Health-AARP Diet and Health Study. Am. J. Clin. Nutr. 88, 431–440 (2008).
Heinen, M. M. et al. Glycemic load, glycemic index, and pancreatic cancer risk in the Netherlands Cohort Study. Am. J. Clin. Nutr. 87, 970–977 (2008).
Gnagnarella, P., Gandini, S., La Vecchia, C. & Maisonneuve, P. Glycemic index, glycemic load, and cancer risk: a meta-analysis. Am. J. Clin. Nutr. 87, 1793–1801 (2008).
Larsson, S. C., Orsini, N. & Wolk, A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int. J. Cancer 120, 1993–1998 (2007).
Renehan, A. G., Tyson, M., Egger, M., Heller, R. F. & Zwahlen, M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371, 569–578 (2008).
Li, D. et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA 301, 2553–1562 (2209).
Berrington de González, A. et al. Anthropometry, physical activity, and the risk of pancreatic cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol. Biomarkers Prev. 15, 879–885 (2006).
Stolzenberg-Solomon, R. Z. et al. Adiposity, physical activity, and pancreatic cancer in the National Institutes of Health-AARP Diet and Health Cohort. Am. J. Epidemiol. 167, 586–597 (2008).
Calton, B. A. et al. A prospective study of physical activity and the risk of pancreatic cancer among women (United States). BMC Cancer 8, 63 (2008).
Stevens, R. J. et al. Factors associated with incident and fatal pancreatic cancer in a cohort of middle-aged women. Int. J. Cancer 124, 2400–2405 (2009).
Anderson, K. E., Johnson, T. W., Lazovich, D. & Folsom, A. R. Association between nonsteroidal anti-inflammatory drug use and the incidence of pancreatic cancer. J. Natl Cancer Inst. 94, 1168–1171 (2002).
Capurso, G. et al. Meta-analysis: the use of non-steroidal anti-inflammatory drugs and pancreatic cancer risk for different exposure categories. Aliment. Pharmacol. Ther. 26, 1089–1099 (2007).
Coogan, P. F. et al. Nonsteroidal anti-inflammatory drugs and risk of digestive cancers at sites other than the large bowel. Cancer Epidemiol. Biomarkers Prev. 9, 119–123 (2000).
Jacobs, E. J. et al. Aspirin use and pancreatic cancer mortality in a large United States cohort. J. Natl Cancer Inst. 96, 524–528 (2004).
Larsson, S. C., Giovannucci, E., Bergkvist, L. & Wolk, A. Aspirin and nonsteroidal anti-inflammatory drug use and risk of pancreatic cancer: a meta-analysis. Cancer Epidemiol. Biomarkers Prev. 15, 2561–2564 (2006).
Menezes, R. J., Huber, K. R., Mahoney, M. C. & Moysich, K. B. Regular use of aspirin and pancreatic cancer risk. BMC Public Health 2, 18 (2002).
Schernhammer, E. S. et al. A prospective study of aspirin use and the risk of pancreatic cancer in women. J. Natl Cancer Inst. 96, 22–28 (2004).
Sumi, S. et al. Inhibition of pancreatic adenocarcinoma cell growth by lovastatin. Gastroenterology 103, 982–989 (1992).
Bonovas, S., Filioussi, K. & Sitaras, N. M. Statins are not associated with a reduced risk of pancreatic cancer at the population level, when taken at low doses for managing hypercholesterolemia: evidence from a meta-analysis of 12 studies. Am. J. Gastroenterol. 103, 2646–2651 (2008).
Luo, J. et al. Body mass index, physical activity and the risk of pancreatic cancer in relation to smoking status and history of diabetes: a large-scale population-based cohort study in Japan—the JPHC study. Cancer Causes Control 18, 603–612 (2007).
Hassan, M. M. et al. Risk factors for pancreatic cancer: case-control study. Am. J. Gastroenterol. 102, 2696–2707 (2007).
Huxley, R., Ansary-Moghaddam, A., Berrington de González, A., Barzi, F. & Woodward, M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br. J. Cancer 92, 2076–2083 (2005).
Chari, S. T. et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology 134, 95–101 (2008).
Li, D., Yeung, S. C., Hassan, M. M., Konopleva, M. & Abbruzzese, J. L. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 137, 482–488 (2009).
Lowenfels, A. B. et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N. Engl. J. Med. 328, 1433–1437 (1993).
Malka, D. et al. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut 51, 849–852 (2002).
Hart, A. R., Kennedy, H. & Harvey, I. Pancreatic cancer: a review of the evidence on causation. Clin. Gastroenterol. Hepatol. 6, 275–282 (2008).
Lowenfels, A. B. et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J. Natl Cancer Inst. 89, 442–446 (1997).
Howes, N. et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin. Gastroenterol. Hepatol. 2, 252–261 (2004).
Rebours, V. et al. Risk of pancreatic adenocarcinoma in patients with hereditary pancreatitis: a national exhaustive series. Am. J. Gastroenterol. 103, 111–119 (2008).
Anderson, L. N., Cotterchio, M. & Gallinger, S. Lifestyle, dietary, and medical history factors associated with pancreatic cancer risk in Ontario, Canada. Cancer Causes Control doi: 10.1007/s10552-009-9303–9305
Gandini, S., Lowenfels, A. B., Jaffee, E. M., Armstrong, T. D. & Maisonneuve, P. Allergies and the risk of pancreatic cancer: a meta-analysis with review of epidemiology and biological mechanisms. Cancer Epidemiol. Biomarkers Prev. 14, 1908–1916 (2005).
Olson, S. H. et al. Allergies, variants in IL-4 and IL-4R alpha genes, and risk of pancreatic cancer. Cancer Detect. Prev. 31, 345–351 (2007).
Turner, M. C., Chen, Y., Krewski, D. & Ghadirian, P. An overview of the association between allergy and cancer. Int. J. Cancer 118, 3124–3132 (2006).
Sherman, P. W., Holland, E. & Sherman, J. S. Allergies: their role in cancer prevention. Q. Rev. Biol. 83, 339–362 (2008).
Farrow, D. C. & Davis, S. Risk of pancreatic cancer in relation to medical history and the use of tobacco, alcohol and coffee. Int. J. Cancer 45, 816–820 (1990).
Stolzenberg-Solomon, R. Z., Pietinen, P., Taylor, P. R., Virtamo, J. & Albanes, D. A. prospective study of medical conditions, anthropometry, physical activity, and pancreatic cancer in male smokers (Finland). Cancer Causes Control 13, 417–426 (2002).
Meyer, M. S., Joshipura, K., Giovannucci, E. & Michaud, D. S. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control 19, 895–907 (2008).
El-Serag, H. B. et al. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U. S. veterans. Hepatology 49, 116–123 (2009).
Hassan, M. M. et al. Association between hepatitis B virus and pancreatic cancer. J. Clin. Oncol. 26, 4557–4562 (2008).
Raderer, M. et al. Association between Helicobacter pylori infection and pancreatic cancer. Oncology 55, 16–19 (1998).
Stolzenberg-Solomon, R. Z. et al. Helicobacter pylori seropositivity as a risk factor for pancreatic cancer. J. Natl Cancer Inst. 93, 937–941 (2001).
Lindkvist, B., Johansen, D., Borgstrom, A. & Manjer, J. A prospective study of Helicobacter pylori in relation to the risk for pancreatic cancer. BMC Cancer 8, 321 (2008).
Wolpin, B. M. et al. ABO blood group and the risk of pancreatic cancer. J. Natl Cancer Inst. 101, 424–431 (2009).
Landi, S. Genetic predisposition and environmental risk factors to pancreatic cancer: A review of the literature. Mutat. Res. 681, 299–307 (2009).
Goldstein, A. M., Struewing, J. P., Fraser, M. C., Smith, M. W. & Tucker, M. A. Prospective risk of cancer in CDKN2A germline mutation carriers. J. Med. Genet. 41, 421–424 (2004).
Vasen, H. F. et al. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden). Int. J. Cancer 87, 809–811 (2000).
De Snoo, F. A. et al. Increased risk of cancer other than melanoma in CDKN2A founder mutation (p16-Leiden)-positive melanoma families. Clin. Cancer Res. 14, 7151–7157 (2008).
Goldstein, A. M. et al. High-risk melanoma susceptibility genes and pancreatic cancer, neural system tumors, and uveal melanoma across GenoMEL. Cancer Res. 66, 9818–9828 (2006).
Howes, N. et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin. Gastroenterol. Hepatol. 2, 252–261 (2004).
Lowenfels, A. B., Maisonneuve, P., Whitcomb, D. C., Lerch, M. M. & DiMagno, E. P. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA 286, 169–170 (2001).
Giardiello, F. M. et al. Very high risk of cancer in familial Peutz–Jeghers syndrome. Gastroenterology 119, 1447–1453 (2000).
Hearle, N. et al. Frequency and spectrum of cancers in the Peutz–Jeghers syndrome. Clin. Cancer Res. 12, 3209–3215 (2006).
Lim, W. et al. Relative frequency and morphology of cancers in STK11 mutation carriers. Gastroenterology 126, 1788–1794 (2004).
Pogue-Geile, K. L. et al. Palladin mutation causes familial pancreatic cancer and suggests a new cancer mechanism. PLoS Med. 3, e516 (2006).
Klein, A. P. et al. Absence of deleterious palladin mutations in patients with familial pancreatic cancer. Cancer Epidemiol. Biomarkers Prev. 18, 1328–1330 (2009).
McWilliams, R. et al. Cystic fibrosis transmembrane regulator gene carrier status is a risk factor for young onset pancreatic adenocarcinoma. Gut 54, 1661–1662 (2005).
Maisonneuve, P., Marshall, B. C. & Lowenfels, A. B. Risk of pancreatic cancer in patients with cystic fibrosis. Gut 56, 1327–1328 (2007).
Malats, N. et al. Cystic fibrosis transmembrane regulator (CFTR) DeltaF508 mutation and 5T allele in patients with chronic pancreatitis and exocrine pancreatic cancer. PANKRAS II Study Group. Gut 48, 70–74 (2001).
Hahn, S. A. et al. BRCA2 germline mutations in familial pancreatic carcinoma. J. Natl Cancer Inst. 95, 214–221 (2003).
van Asperen, C. J. et al. Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. J. Med. Genet. 42, 711–719 (2005).
Risch, H. A. et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J. Natl Cancer Inst. 98, 1694–1706 (2006).
Couch, F. J. et al. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol. Biomarkers Prev. 16, 342–346 (2007).
Ferrone, C. R. et al. BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma. J. Clin. Oncol. 27, 433–438 (2009).
Cho, J. H., Bang, S., Park, S. W., Chung, J. B. & Song, S. Y. BRCA2 mutations as a universal risk factor for pancreatic cancer has a limited role in Korean ethnic group. Pancreas 36, 337–340 (2008).
Real, F. X. et al. Family history of cancer and germline BRCA2 mutations in sporadic exocrine pancreatic cancer. Gut 50, 653–657 (2002).
Jones, S. et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science 324, 217 (2009).
Giardiello, F. M. et al. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut 34, 1394–1396 (1993).
Kleihues, P., Schauble, B., zur Hausen, A., Esteve, J. & Ohgaki, H. Tumors associated with p53 germline mutations: a synopsis of 91 families. Am. J. Pathol. 150, 1–13 (1997).
Geary, J. et al. Gene-related cancer spectrum in families with hereditary non-polyposis colorectal cancer (HNPCC). Fam. Cancer 7, 163–172 (2008).
Maitra, A. & Hruban, R. H. Pancreatic cancer. Annu. Rev. Pathol. 3, 157–188 (2008).
Su, G. H. et al. Germline and somatic mutations of the STK11/LKB1 Peutz–Jeghers gene in pancreatic and biliary cancers. Am. J. Pathol. 154, 1835–1840 (1999).
Blackford, A. et al. Genetic mutations associated with cigarette smoking in pancreatic cancer. Cancer Res. 69, 3681–3688 (2009).
Crous-Bou, M. et al. Lifetime history of alcohol consumption and K-ras mutations in pancreatic ductal adenocarcinoma. Environ. Mol. Mutagen. 50, 421–430 (2009).
Morales, E. et al. Food and nutrient intakes and K-ras mutations in exocrine pancreatic cancer. J. Epidemiol. Community Health 61, 641–649 (2007).
Jiao, L. et al. Glutathione S-transferase gene polymorphisms and risk and survival of pancreatic cancer. Cancer 109, 840–848 (2007).
Li, D. et al. Polymorphisms of cytochrome P4501A2 and N-acetyltransferase genes, smoking, and risk of pancreatic cancer. Carcinogenesis 27, 103–111 (2006).
Ayaz, L., Ercan, B., Dirlik, M., Atik, U. & Tamer, L. The association between N-acetyltransferase 2 gene polymorphisms and pancreatic cancer. Cell. Biochem. Funct. 26, 329–333 (2008).
Ockenga, J., Vogel, A., Teich, N., Keim, V., Manns, M. P. & Strassburg, C. P. UDP glucuronosyltransferase (UGT1A7) gene polymorphisms increase the risk of chronic pancreatitis and pancreatic cancer. Gastroenterology 124, 1802–1808 (2003).
Suzuki, H. et al. Interaction of the cytochrome P4501A2, SULT1A1 and NAT gene polymorphisms with smoking and dietary mutagen intake in modification of the risk of pancreatic cancer. Carcinogenesis 29, 1184–1191 (2008).
Duell, E. J. et al. A population-based, case-control study of polymorphisms in carcinogen-metabolizing genes, smoking, and pancreatic adenocarcinoma risk. J. Natl Cancer Inst. 94, 297–306 (2002).
Li, D. et al. 5, 10-Methylenetetrahydrofolate reductase polymorphisms and the risk of pancreatic cancer. Cancer Epidemiol. Biomarkers Prev. 14, 1470–1476 (2005).
Wang, L. et al. Genetic polymorphisms in methylenetetrahydrofolate reductase and thymidylate synthase and risk of pancreatic cancer. Clin. Gastroenterol. Hepatol. 3, 743–751 (2005).
Nisevic, I. et al. MTHFR C677T polymorphism in chronic pancreatitis and pancreatic adenocarcinoma. Cell. Biochem. Funct. 26, 659–663 (2008).
Suzuki, T. et al. Alcohol drinking and one-carbon metabolism-related gene polymorphisms on pancreatic cancer risk. Cancer Epidemiol. Biomarkers Prev. 17, 2742–2747 (2008).
Li, D. et al. DNA repair gene polymorphisms and risk of pancreatic cancer. Clin. Cancer Res. 15, 740–746 (2009).
McWilliams, R. R. et al. Polymorphisms in DNA repair genes, smoking, and pancreatic adenocarcinoma risk. Cancer Res. 68, 4928–4935 (2008).
Duell, E. J. et al. Detecting pathway-based gene-gene and gene–environment interactions in pancreatic cancer. Cancer Epidemiol. Biomarkers Prev. 17, 1470–1479 (2008).
Jiao, L. et al. XRCC2 and XRCC3 gene polymorphism and risk of pancreatic cancer. Am. J. Gastroenterol. 103, 360–367 (2008).
Acknowledgements
The authors were supported by grants from the C. D. Smithers Foundation and Solvay Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Raimondi, S., Maisonneuve, P. & Lowenfels, A. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol 6, 699–708 (2009). https://doi.org/10.1038/nrgastro.2009.177
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2009.177
This article is cited by
-
Liquid biopsy techniques and pancreatic cancer: diagnosis, monitoring, and evaluation
Molecular Cancer (2023)
-
TWIST1 is a prognostic factor for neoadjuvant chemotherapy for patients with resectable pancreatic cancer: a preliminary study
Surgery Today (2023)
-
Risk factors related to age at diagnosis of pancreatic cancer: a retrospective cohort pilot study
BMC Gastroenterology (2022)
-
Development of a prognostic nomogram for metastatic pancreatic ductal adenocarcinoma integrating marital status
Scientific Reports (2022)
-
Surgical and oncological outcomes of laparoscopic versus open radical antegrade modular pancreatosplenectomy for pancreatic ductal adenocarcinoma
Surgery Today (2022)