Abstract
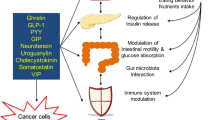
Excess body weight (EBW) is an independent risk factor for many human malignancies, including cancers throughout the gastrointestinal and hepatobiliary tract from the esophagus to the colorectum. The relative risk of gastrointestinal cancer in obese individuals is approximately 1.5–2.0 times that for normal weight individuals, with organ-specific and gender-specific differences for specific cancers. The association between EBW and risk of premalignant stages of gastrointestinal carcinogenesis, such as colorectal adenoma and Barrett esophagus, is similar, implying a role for EBW during the early stages of carcinogenesis that could be relevant to preventative strategies. EBW also impacts negatively on gastrointestinal cancer outcomes. The mechanistic basis of the association between EBW and carcinogenesis remains incompletely understood. Postulated mechanisms include increased insulin and insulin-like growth factor signaling and chronic inflammation (both linked to the metabolic syndrome), as well as signaling via adipokines, such as leptin. The role of obesity-related changes in the intestinal microbiome in gastrointestinal carcinogenesis deserves further attention. Whether weight loss leads to reduced future gastrointestinal and liver cancer risk has yet to be fully explored. There is some support for the idea that weight loss negatively regulates colorectal carcinogenesis. In addition, data suggest a reduction in risk of several cancers in the first 10 years after bariatric surgery.
Key Points
-
Excess body weight (EBW) is a potentially preventable cause of several gastrointestinal and hepatobiliary malignancies, including colorectal, esophageal, liver and pancreatic cancer
-
A positive association between EBW and premalignant conditions, such as colorectal adenoma and Barrett esophagus, implies that EBW promotes the early stages of carcinogenesis (which could be relevant for prevention)
-
The mechanisms linking excess adiposity (particularly visceral or abdominal fat) and gastrointestinal carcinogenesis are unclear but may include insulin and insulin-like growth factor signaling, chronic inflammation, adipokine signaling and changes to the intestinal microbiome
-
Whether weight loss reduces future gastrointestinal and liver cancer risk has not been addressed fully and requires further study
-
EBW is also associated with worse outcomes from gastrointestinal cancer following diagnosis
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Moghaddam, A. A., Woodward, M. & Huxley, R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol. Biomarkers Prev. 16, 2533–2547 (2007).
Renehan, A. G., Tyson, M., Egger, M., Heller, R. F. & Zwahlen, M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371, 569–578 (2008).
WHO. Obesity and overweight. Fact Sheet No 311. World Health Organization [online], (2006).
World Cancer Research Fund. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective 2nd edn (American Institute for Cancer Research, Washington, 2007).
Vainoio, H. & Bianchinin, F. (Eds) International Agency for Research in Cancer. Weight control and physical activity. (IARC Press, Lyon, 2002).
Renehan, A. G. et al. Incident cancer burden attributable to excess body mass index in 30 European countries. Int. J. Cancer 126, 692–702 (2010).
Wolk, A. et al. A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control 12, 13–21 (2001).
Siddiqui, A. et al. Obesity is associated with an increased prevalence of advanced adenomatous colon polyps in a male veteran population. Dig. Dis. Sci. 54, 1560–1564 (2009).
Jacobson, B. C., Chan, A. T., Giovannucci, E. L. & Fuchs, C. S. Body mass index and Barrett's oesophagus in women. Gut 58, 1460–1466 (2009).
Sato, Y., Nozaki, R., Yamada, K., Takano, M. & Haruma, K. Relation between obesity and adenomatous polyps of the large bowel. Dig. Endosc. 21, 154–157 (2009).
Cook, M. B., Greenwood, D. C., Hardie, L. J., Wild, C. R. & Forman, D. A systematic review and meta-analysis of the risk of increasing adiposity on Barrett's esophagus. Am. J. Gastroenterol. 103, 292–300 (2008).
Thygesen, L. C. et al. Prospective weight change and colon cancer risk in male US health professionals. Int. J. Cancer 123, 1160–1165 (2008).
National Institutes for Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. The evidence report. (NHLBI, 1998).
Larsson, S. C. & Wolk, A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am. J. Clin. Nutr. 86, 556–565 (2007).
Dai, Z., Xu, Y. C. & Niu, L. Obesity and colorectal cancer risk: a meta-analysis of cohort studies. World J. Gastroenterol. 13, 4199–4206 (2007).
Moore, L. L. et al. BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham Study adults. Int. J. Obes. 28, 559–567 (2004).
Wise, L. A., Rosenberg, L., Palmer, J. R. & Adams-Campbell, L. L. Anthropometric risk factors for colorectal polyps in African-American women. Obesity 16, 859–868 (2008).
Sedjo, R. L. et al. Change in body size and the risk of colorectal adenomas. Cancer Epidemiol. Biomarkers Prev. 16, 526–531 (2007).
Larsen, I. K., Grotmol, T., Almendingen, K. & Hoff, G. Lifestyle as a predictor for colonic neoplasia in asymptomatic individuals. BMC Gastroenterol. 6, 5 (2006).
Neugut, A. I. et al. Obesity and colorectal adenomatous polyps. J. Natl Cancer Inst. 83, 359–361 (1991).
Morois, S., Mesrine, S., Josset, M., Clavel-Chapelon, F. & Boutron-Ruault, M.-C. Anthropometric factors in adulthood and risk of colorectal adenomas. Am. J. Epidemiol. 172, 1166–1180 (2010).
Giovannucci, E. et al. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann. Intern. Med. 122, 327–334 (1995).
Giovannucci, E., Colditz, G. A., Stampfer, M. J. & Willett, W. C. Physical activity obesity and risk of colorectal adenoma in women (United States). Cancer Causes Control 7, 253–263 (1996).
Yamaji, Y. et al. The effect of body weight reduction on the incidence of colorectal adenoma. Am. J. Gastroenterol. 103, 2061–2067 (2008).
Lieberman, D. A., Prindiville, S., Weiss, D. G. & Willett, W. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA 290, 2959–2967 (2003).
Anderson, J. C. et al. Body mass index: a marker for significant colorectal neoplasia in a screening population. J. Clin. Gastroenterol. 41, 285–290 (2007).
Omata, F. et al. Modifiable risk factors for colorectal neoplasms and hyperplastic polyps. Intern. Med. 48, 123–128 (2009).
Kim, Y. & Lee, S. An association between colonic adenoma and abdominal obesity: a cross-sectional study. BMC Gastroenterol. 9, 4 (2009).
Betes, M. et al. Use of colonoscopy as a primary screening test for colorectal cancer in average risk people. Am. J. Gastroenterol. 98, 2648–2654 (2003).
Bird, C. L., Frankl, H. D., Lee, E. R. & Haile, R. W. Obesity, weight gain, large weight changes, and adenomatous polyps of the left colon and rectum. Am. J. Epidemiol. 147, 670–680 (1998).
Kono, S., Shinchi, K. & Imanishi, K. Body mass index and adenomas of the sigmoid colon in Japanese men. Eur. J. Epidemiol. 12, 425–426 (1996).
Boutron-Ruault, M.-C., Senesse, P., Méance, S., Belghiti, C. & Faivre, J. Energy intake, body mass index, physical activity, and the colorectal adenoma-carcinoma sequence. Nutr. Cancer 39, 50–57 (2001).
Wallace, K. et al. The association of physical activity and body mass index with the risk of large bowel polyps. Cancer Epidemiol. Biomarkers Prev. 14, 2082–2086 (2005).
Terry, M. B. et al. Risk factors for advanced colorectal adenomas: a pooled analysis. Cancer Epidemiol. Biomarkers Prev. 11, 622–629 (2002).
Wolf, L. A., Terry, P. D., Potter, J. D. & Bostick, R. M. Do factors related to endogenous and exogenous estrogens modify the relationship between obesity and risk of colorectal adenomas in women? Cancer Epidemiol. Biomarkers Prev. 16, 676–683 (2007).
Almendingen, K., Hofstad, B. & Vatn, M. H. Does high body fatness increase the risk of presence and growth of colorectal adenomas followed up in situ for 3 years? Am. J. Gastroenterol. 96, 2238–2246 (2001).
Bayerdörffer, E. et al. Increased risk of 'high-risk' colorectal adenomas in overweight men. Gastroenterology 104, 137–144 (1993).
Kang, H. W. et al. Visceral obesity and insulin resistance as risk factors for colorectal adenoma: a cross-sectional, case-control study. Am. J. Gastroenterol. 105, 178–187 (2010).
Kim, J. H. et al. Is metabolic syndrome a risk factor for colorectal adenoma? Cancer Epidemiol. Biomarkers Prev. 16, 1543–1546 (2007).
Kim, K. S. et al. The frequency and risk factors of colorectal adenoma in health-check-up subjects in South Korea: relationship to abdominal obesity and age. Gut Liver 4, 36–42 (2010).
Jacobs, E. T. et al. Association between body mass index and colorectal neoplasia at follow-up colonoscopy: a pooling study. Am. J. Epidemiol. 169, 657–666 (2009).
Sainsbury, A. et al. Increased colorectal epithelial cell proliferation and crypt fission associated with obesity and roux-en-Y gastric bypass. Cancer Epidemiol. Biomarkers Prev. 17, 1401–1410 (2008).
Ferrante, J. M. et al. Colorectal cancer screening among obese versus non-obese patients in primary care practices. Cancer Detect. Prev. 30, 459–465 (2006).
Rosen, A. B. & Schneider, E. C. Colorectal cancer screening disparities related to obesity and gender. J. Gen. Intern. Med. 19, 332–338 (2004).
Borg, B. B., Gupta, N. K., Zuckerman, G. R., Banerjee, B. & Gyawali, C. P. Impact of obesity on bowel preparation for colonoscopy. Clin. Gastroenterol. Hepatol. 7, 670–675 (2009).
Pera, M., Manterola, C., Vidal, O. & Grande, L. Epidemiology of esophageal adenocarcinoma. J. Surg. Onc. 92, 151–159 (2005).
Lagergren, J., Bergstrom, R., Lindgren, A. & Nyren, O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N. Engl. J. Med. 340, 825–831 (1999).
Hampel, H., Abraham, N. S. & El-Serag, H. B. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann. Intern. Med. 143, 199–211 (2005).
Corley, D. A. & Kubo, A. Body mass index and gastroesophageal reflux disease: a systematic review and meta-analysis. Am. J. Gastroenterol. 101, 2619–2628 (2006).
Corley, D. A., Kubo, A. & Zhao, W. Abdominal obesity, ethnicity and gastro-oesophageal reflux symptoms. Gut 56, 756–762 (2007).
Whiteman, D. et al. The role of obesity, gastroesophageal reflux and smoking in adenocarcinomas of the esophagus and esophago-gastric junction [abstract]. Gastroenterology 132, A418 (2007).
Yousef, F. et al. The incidence of esophageal cancer and high-grade dysplasia in Barrett's esophagus: a systematic review and meta-analysis. Am. J. Epidemiol. 168, 237–249 (2008).
Kamat, P., Wen, S. J., Morris, J. & Anandasabapathy, S. Exploring the association between elevated body mass index and Barrett's esophagus: a systematic review and meta-analysis. Ann. Thorac. Surg. 87, 655–662 (2009).
Edelstein, Z. R., Farrow, D. C., Bronner, M. P., Rosen, S. N. & Vaughan, T. L. Central adiposity and risk of Barrett's esophagus. Gastroenterology 133, 403–411 (2007).
Corley, D. A. et al. Abdominal obesity and body mass index as risk factors for Barrett's esophagus. Gastroenterology 133, 34–41 (2007).
Oberg, S., Wenner, J., Johansson, J., Walther, B. & Willen, R. Barrett esophagus: risk factors for progression to dysplasia and adenocarcinoma. Ann. Surg. 242, 49–54 (2005).
Arslan, A. A. et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch. Intern. Med. 170, 791–802 (2010).
Larsson, S. C., Orsini, N. & Wolk, A. Body mass index and pancreatic cancer risk: a meta-analysis of prospective studies. Int. J. Cancer 120, 1993–1998 (2007).
Li, D. et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA 301, 2553–2562 (2009).
Huxley, R., Ansary-Moghaddam, A., de Gonzalez, A. B., Barzi, F. & Woodward, M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br. J. Cancer 92, 2076–2083 (2005).
Permert, J. et al. Is profound peripheral insulin resistance in patients with pancreatic cancer caused by a tumor-associated factor? Am. J. Surg. 165, 61–66 (1993).
Lowenfels, A. B. et al. Pancreatitis and the risk of pancreatic cancer. N. Engl. J. Med. 328, 1433–1437 (1993).
Yang, P. et al. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur. J. Cancer 45, 2867–2873 (2009).
Leung, W. K. et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 9, 279–287 (2008).
Goldblum, J. R. et al. Helicobacter pylori infection, not gastroesophageal reflux, is the major cause of inflammation and intestinal metaplasia of gastric cardiac mucosa. Am. J. Gastroenterol. 97, 302–311 (2002).
Kelley, J. R. & Duggan, J. M. Gastric cancer epidemiology and risk factors. J. Clin. Epidemiol. 56, 1–9 (2003).
Wistuba, I. I. & Gazdar, A. F. Gallbladder cancer: Lessons from a rare tumour. Nat. Rev. Cancer 4, 695–706 (2004).
Engeland, A., Tretli, S., Austad, G. & Bjorge, T. Height and body mass index in relation to colorectal and gallbladder cancer in two million Norwegian men and women. Cancer Causes Control 16, 987–996 (2005).
Hsing, A. W. et al. Body size and the risk of biliary tract cancer: a population-based study in China. Br. J. Cancer 99, 811–815 (2008).
Stampfer, M. J., Maclure, K. M., Colditz, G. A., Manson, J. E. & Willett, W. C. Risk of symptomatic gallstones in women with severe obesity. Am. J. Clin. Nutr. 55, 652–658 (1992).
Lazcano-Ponce, E. C. et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J. 51, 349–364 (2001).
Warren, B. L. & Marais, A. W. Carcinoma of the gallbladder: a possible regional predisposition in the western Cape and the northern Cape. S. Afr. J. Surg. 33, 161–164 (1995).
Shrikhande, S. V., Barreto, S. G., Singh, S., Udwadia, T. E. & Agarwal, A. K. Cholelithiasis in gallbladder cancer: coincidence, cofactor, or cause! Eur. J. Surg. Oncol. 36, 514–519 (2010).
Randi, G., Franceschi, S. & La Vecchia, C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int. J. Cancer 118, 1591–1602 (2006).
Mohandas, K. M. & Patil, P. S. Cholecystectomy for asymptomatic gallstones can reduce gall bladder cancer mortality in northern Indian women. Ind. J. Gastroenterol. 25, 147–151 (2006).
Duarte, I., Llanos, O., Domke, H., Harz, C. & Valdivieso, V. Metaplasia and precursor lesions of gallbladder carcinoma. Frequency, distribution, and probability of detection in routine histologic samples. Cancer 72, 1878–1884 (1993).
Lim, S. H. et al. Is metabolic syndrome one of the risk factors for gallbladder polyps Found by ultrasonography during health screening? Gut Liver 1, 138–144 (2007).
Segawa, K. et al. Prevalence of gallbladder polyps among apparently healthy Japanese: ultrasonographic study. Am. J. Gastroenterol. 87, 630–633 (1992).
Liew, P. L. et al. Gallbladder disease among obese patients in Taiwan. Obes. Surg. 17, 383–390 (2007).
Patel, T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2, 10 (2002).
Grainge, M. J., West, J., Solaymani-Dodaran, M., Aithal, G. P. & Card, T. R. The antecedents of biliary cancer: a primary care case-control study in the United Kingdom. Br. J. Cancer 100, 178–180 (2008).
Welzel, T. M. et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin. Gastroenterol. Hepatol. 5, 1221–1228 (2007).
Welzel, T. M. et al. Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case-control study. Int. J. Cancer 120, 638–641 (2007).
Starley, B. Q., Calcagno, C. J. & Harrison, S. A. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 51, 1820–1832 (2010).
Liu, B., Balkwill, A., Reeves, G. & Beral, V. Body mass index and risk of liver cirrhosis in middle aged UK women: prospective study. BMJ 340, c912 (2010).
Omagari, K. et al. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J. Gastroenterol. Hepatol. 17, 1098–1105 (2002).
Browning, J. D. et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40, 1387–1395 (2004).
Machado, M., Marques-Vidal, P. & Cortez-Pinto, H. Hepatic histology in obese patients undergoing bariatric surgery. J. Hepatol. 45, 600–606 (2006).
Saunders, D., Seidel, D., Allison, M. & Lyratzopoulos, G. Systematic review: the association between obesity and hepatocellular carcinoma—epidemiological evidence. Aliment. Pharmacol. Ther. 31, 1051–1063 (2010).
Larsson, S. C. & Wolk, A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br. J. Cancer 97, 1005–1008 (2007).
Oh, S. W., Yoon, Y. S. & Shin, S. A. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J. Clin. Oncol. 23, 4742–4754 (2005).
Nair, S., Mason, A., Eason, J., Loss, G. & Perrillo, R. P. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology 36, 150–155 (2002).
Ohki, T. et al. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clin. Gastroenterol. Hepatol. 6, 459–464 (2008).
Park, E. J. et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140, 197–208 (2010).
Ichikawa, T. et al. Two cases of non-alcoholic steatohepatitis with development of hepatocellular carcinoma without cirrhosis. J. Gastroenterol. Hepatol. 21, 1865–1866 (2006).
Kawada, N. et al. Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J. Gastroenterol. 44, 1190–1194 (2009).
Wellen, K. E. & Hotamisligil, G. S. Inflammation, stress, and diabetes. J. Clin. Invest. 115, 1111–1119 (2005).
Roberts, D. L., Dive, C. & Renehan, A. G. Biological mechanisms linking obesity and cancer risk: new perspectives. Ann. Rev. Med. 61, 301–316 (2010).
Lin, J. H. & Giovannucci, E. Sex hormones and colorectal cancer: what have we learned so far? J. Natl Cancer Inst. 102, 1746–1747 (2010).
Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023 (2006).
Caesar, R., Fåk, F. & Bäckhed, F. Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism. J. Int. Med. 268, 320–328 (2010).
Cowey, S. & Hardy, R. W. The metabolic syndrome: a high-risk state for cancer? Am. J. Pathol. 169, 1505–1522 (2006).
Donohoe, C. L., Pidgeon, G. P., Lysaght, J. & Reynolds, J. V. Obesity and gastrointestinal cancer. Br. J. Surg. 97, 628–642 (2010).
Renehan, A. G., Frystyk, J. & Flyvbjerg, A. Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrinol. Metab. 17, 328–336 (2006).
Larsson, S. C., Giovannucci, E. & Wolk, A. Diabetes and colorectal cancer incidence in the cohort of Swedish men. Diabetes Care 28, 1805–1807 (2005).
Clayton, P. E., Banerjee, I., Murray, P. G. & Renehan, A. G. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat. Rev. Endocrinol. 7, 11–24 (2011).
Giovannucci, E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J. Nutr. 131, 3109S–3120S (2001).
Corpet, D. E., Jacquinet, C., Peiffer, G. & Tache, S. Insulin injections promote the growth of aberrant crypt foci in the colon of rats. Nutr. Cancer 27, 316–320 (1997).
Samani, A. A., Yakar, S., LeRoith, D. & Brodt, P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr. Rev. 28, 20–47 (2007).
Sears, R. et al. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 14, 2501–2514 (2000).
Hursting, S. D. et al. Reducing the weight of cancer: mechanistic targets for breaking the obesity-carcinogenesis link. Best Pract. Res. Clin. Endocrinol. Metab. 22, 659–669 (2008).
Hosono, K. et al. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev. Res. 3, 1077–1083 (2010).
Tomimoto, A. et al. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci. 99, 2136–2141 (2008).
Renehan, A. G. et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 363, 1346–1353 (2004).
Schoen, R. E. et al. Insulin-like growth factor-I and insulin are associated with the presence and advancement of adenomatous polyps. Gastroenterology 129, 464–475 (2005).
Renehan, A. G. et al. High-risk colorectal adenomas and serum insulin-like growth factors. Br. J. Surg. 88, 107–113 (2001).
Flood, A. et al. Serum concentrations of insulin-like growth factor and insulin-like growth factor binding protein 3 and recurrent colorectal adenomas. Cancer Epidemiol. Biomarkers Prev. 17, 1493–1498 (2008).
Wolpin, B. M. et al. Circulating insulin-like growth factor binding protein-1 and the risk of pancreatic cancer. Cancer Res. 67, 7923–7928 (2007).
Bergmann, U., Funatomi, H., Yokoyama, M., Beger, H. G. & Korc, M. Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer Res. 55, 2007–2011 (1995).
Zhao, R., Macdonald, K. & Casson, A. G. Insulin-like growth factor type I receptor gene expression and obesity in esophageal adenocarcinoma. Mol. Carcinog. 48, 982–988 (2009).
Hoyo, C. et al. IGF2R polymorphisms and risk of esophageal and gastric adenocarcinomas. Int. J. Cancer 125, 2673–2678 (2009).
McElholm, A. R. et al. A population-based study of IGF axis polymorphisms and the esophageal inflammation, metaplasia, adenocarcinoma sequence. Gastroenterology 139, 204–212.e3 (2010).
Park, H. S., Park, J. Y. & Yu, R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-[alpha] and IL-6. Diab. Res. Clin. Pract. 69, 29–35 (2005).
Nishimura, S. et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920 (2009).
Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15, 930–939 (2009).
Balistreri, C. R., Caruso, C. & Candore, G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediators Inflamm. 2010, 802078 (2010).
Song, M. J., Kim, K. H., Yoon, J. M. & Kim, J. B. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem. Biophys. Res. Commun. 346, 739–745 (2006).
Balkwill, F., Charles, K. A. & Mantovani, A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7, 211–217 (2005).
Poullis, A. Bowel inflammation as measured by faecal calprotectin: a link between lifestyle factors and colorectal cancer risk. Cancer Epidemiol. Biomarkers Prev. 13, 279–284 (2004).
Pendyala, S., Neff, L. M., Suárez-Fariñas, M. & Holt, P. R. Diet-induced weight loss reduces colorectal inflammation: implications for colorectal carcinogenesis. Am. J. Clin. Nutr. 93, 234–242 (2011).
Martínez, M. E. et al. Physical activity, body mass index, and prostaglandin E2 levels in rectal mucosa. J. Natl Cancer Inst. 91, 950–953 (1999).
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Tsilidis, K. K. et al. C-reactive protein and colorectal cancer risk: a systematic review of prospective studies. Int. J. Cancer 123, 1133–1140 (2008).
Tsilidis, K. K. et al. C-reactive protein and colorectal adenoma in the CLUE II cohort. Cancer Causes Control 19, 559–567 (2008).
Kim, S. et al. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res. 68, 323–328 (2008).
Considine, R. V. et al. Serum immunoreactive leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 334, 292–295 (1996).
Aloulou, N. et al. Involvement of the leptin receptor in the immune response in intestinal cancer. Cancer Res. 68, 9413–9422 (2008).
Liu, Z. H., Uesaka, T., Watanabe, H. & Kato, N. High fat diet enhances colonic cell proliferation and carcinogenesis in rats by elevating serum leptin. Int. J. Oncol. 19, 1009–1014 (2001).
Rouet-Benzineb, P. et al. Leptin counteracts sodium butyrate-induced apoptosis in human colon cancer HT-29 cells via NF-kappaB signaling. J. Biol. Chem. 279, 16495–16502 (2004).
Hirose, Y. et al. Enhancement of development of azoxymethane-induced colonic premalignant lesions in C57BL/KsJ-db/db mice. Carcinogenesis 25, 821–825 (2004).
Ogunwobi, O., Mutungi, G. & Beales, I. L. P. Leptin stimulates proliferation and inhibits apoptosis in Barrett's esophageal adenocarcinoma cells by cyclooxygenase-2-dependent, prostaglandin-E2-mediated transactivation of the epidermal growth factor receptor and c-Jun NH2-terminal kinase activation. Endocrinology 147, 4505–4516 (2006).
Chia, V. M. et al. Leptin concentrations, leptin receptor polymorphisms, and colorectal adenoma risk. Cancer Epidemiol. Biomarkers Prev. 16, 2697–2703 (2007).
Stattin, P. et al. Plasma leptin and colorectal cancer risk: a prospective study in Northern Sweden. Oncol. Rep. 10, 2015–2021 (2003).
Stattin, P. et al. Obesity and colon cancer: does leptin provide a link? Int. J. Cancer 109, 149–152 (2004).
Kendall, B. J. et al. Leptin and the risk of Barrett's oesophagus. Gut 57, 448–454 (2008).
Wang, S. N., Lee, K. T. & Ker, C. G. Leptin in hepatocellular carcinoma. World J. Gastroenterol. 16, 5801–5809 (2010).
Arita, Y. et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 257, 79–83 (1999).
Sugiyama, M. et al. Adiponectin inhibits colorectal cancer cell growth through the AMPK/mTOR pathway. Int. J. Oncol. 34, 339–344 (2009).
Otani, K. et al. Adiponectin suppresses tumorigenesis in Apc(Min/+) mice. Cancer Lett. 288, 177–182 (2010).
Takahashi, H. et al. Association of visceral fat accumulation and plasma adiponectin with rectal dysplastic aberrant crypt foci in a clinical population. Cancer Sci. 100, 29–32 (2009).
Wei, E. K., Giovannucci, E., Fuchs, C. S., Willett, W. C. & Mantzoros, C. S. Low plasma adiponectin levels and risk of colorectal cancer in men: A prospective study. J. Natl Cancer Inst. 97, 1688–1694 (2005).
Nakajima, T. E. et al. Adipocytokines as new promising markers of colorectal tumors: adiponectin for colorectal adenoma, and resistin and visfatin for colorectal cancer. Cancer Sci. 101, 1286–1291 (2010).
Yamaji, T., Iwasaki, M., Sasazuki, S. & Tsugane, S. Interaction between adiponectin and leptin Influences the risk of colorectal adenoma. Cancer Res. 70, 5430–5437 (2010).
Otake, S. et al. Decreased levels of plasma adiponectin associated with increased risk of colorectal cancer. World J. Gastroenterol. 16, 1252–1257 (2010).
Ishikawa, M. et al. Plasma adiponectin and gastric cancer. Clin. Cancer Res. 11, 466–472 (2005).
Otani, K. et al. Adiponectin receptors are downregulated in human gastric cancer. J. Gastroenterol. 45, 918–927 (2010).
Konturek, P. C., Burnat, G., Rau, T., Hahn, E. G. & Konturek, S. Effect of adiponectin and ghrelin on apoptosis of barrett adenocarcinoma cell line. Dig. Dis. Sci. 53, 597–605 (2008).
Sharma, D. et al. Adiponectin antagonizes the oncogenic actions of leptin in hepatocellular carcinogenesis. Hepatology 52, 1713–1722 (2010).
Backhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl Acad. Sci. USA 101, 15718–15723 (2004).
Backhed, F., Manchester, J. K., Semenkovich, C. F. & Gordon, J. I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl Acad. Sci. USA 104, 979–984 (2007).
Furet, J. P. et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 59, 3049–3057 (2010).
Shen, X. J. et al. Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes 1, 138–147 (2010).
Vannucci, L. et al. Colorectal carcinogenesis in germ-free and conventionally reared rats: different intestinal environments affect the systemic immunity. Int. J. Oncol. 32, 609–617 (2008).
Martin, H. M. et al. Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology 127, 80–93 (2004).
Swidsinski, A. et al. Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterology 115, 281–286 (1998).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007).
Parker, E. D. & Folsom, A. R. Intentional weight loss and incidence of obesity-related cancers: the Iowa Women's Health Study. Int. J. Obes. Relat. Metab. Disord. 27, 1447–1452 (2003).
Rapp, K. et al. Weight change and cancer risk in a cohort of more than 65 000 adults in Austria. Ann. Oncol. 19, 641–648 (2008).
St. George, A. et al. Effect of a lifestyle intervention in patients with abnormal liver enzymes and metabolic risk factors. J. Gastroenterol. Hepatol. 24, 399–407 (2009).
Stewart, L. K. et al. The influence of exercise training on inflammatory cytokines and C-reactive protein. Med. Sci. Sports Exerc. 39, 1714–1719 (2007).
Rankin, J. W. & Turpyn, A. D. Low carbohydrate, high fat diet increases C-reactive protein during weight loss. J. Am. Coll. Nutr. 26, 163–169 (2007).
Tammariello, A. E. & Milner, J. A. Mouse models for unraveling the importance of diet in colon cancer prevention. J. Nutr. Biochem. 21, 77–88 (2010).
Johnson, N. A. et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 50, 1105–1112 (2009).
Kirkegaard, H. et al. Association of adherence to lifestyle recommendations and risk of colorectal cancer: a prospective Danish cohort study. BMJ 341, c5504 (2010).
Sjöström, L. et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 10, 653–662 (2009).
Adams, T. D. et al. Cancer incidence and mortality after gastric bypass surgery. Obesity 17, 796–802 (2009).
Christou, N. V., Lieberman, M., Sampalis, F. & Sampalis, J. S. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg. Obes. Relat. Dis. 4, 691–695 (2008).
Plecka-Ostlund, M. Risk of obesity-related cancer after obesity surgery in a population-based cohort study. Ann. Surg. 252, 972–976 (2010).
Prizment, A. E., Flood, A., Anderson, K. E. & Folsom, A. R. Survival of women with colon cancer in relation to precancer anthropometric characteristics: the Iowa Women's Health Study. Cancer Epidemiol. Biomarkers Prev. 19, 2229–2237 (2010).
Haydon, A. M. M., MacInnis, R. J., English, D. R. & Giles, G. G. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut 55, 62–67 (2006).
Wu, Y. J. et al. Insulin-like growth factor-I regulates the liver microenvironment in obese mice and promotes liver metastasis. Cancer Res. 70, 57–67 (2010).
Earl, T. M. et al. Silencing of TLR4 decreases liver tumor burden in a murine model of colorectal metastasis and hepatic steatosis. Ann. Surg. Oncol. 16, 1043–1050 (2009).
Benoist, S., Panis, Y., Alves, A. & Valleur, P. Impact of obesity on surgical outcomes after colorectal resection. Am. J. Surg. 179, 275–281 (2000).
Meyerhardt, J. A. et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from intergroup trial 0114. J. Clin. Oncol. 22, 648–657 (2004).
Moon, H. G. et al. Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann. Surg. Oncol. 15, 1918–1922 (2008).
Meyerhardt, J. A. et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer 98, 484–495 (2003).
Dignam, J. J. et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J. Natl Cancer Inst. 98, 1647–1654 (2006).
Guiu, B. et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut 59, 341–347 (2010).
Grotenhuis, B. A. et al. Prognostic value of body mass index on short-term and long-term outcome after resection of esophageal cancer. World J. Surg. 34, 2621–2627 (2010).
Healy, L. A. et al. Impact of obesity on outcomes in the management of localized adenocarcinoma of the esophagus and esophagogastric junction. J. Thorac. Cardiovasc. Surg. 134, 1284–1291 (2007).
Scipione, C. N., Chang, A. C., Pickens, A., Lau, C. L. & Orringer, M. B. Transhiatal esophagectomy in the profoundly obese: implications and experience. Ann. Thorac. Surg. 84, 376–383 (2007).
Melis, M. et al. An elevated body mass index does not reduce survival after esophagectomy for cancer. Ann. Surg. Oncol. doi:10.1245/s10434-010-1336-1.
Oh, S. J. et al. Effect of being overweight on postoperative morbidity and long-term surgical outcomes in proximal gastric carcinoma. J. Gastroenterol. Hepatol. 24, 475–479 (2009).
Tokunaga, M. et al. Better 5-year survival rate following curative gastrectomy in overweight patients. Ann. Surg. Oncol. 16, 3245–3251 (2009).
Zyromski, N. J. et al. Obesity potentiates the growth and dissemination of pancreatic cancer. Surgery 146, 258–263 (2009).
Noun, R. et al. The impact of obesity on surgical outcome after pancreaticoduodenectomy. JOP 9, 468–476 (2008).
Rosso, E. et al. The role of “fatty pancreas” and of BMI in the occurrence of pancreatic fistula after pancreaticoduodenectomy. J. Gastrointest. Surg. 13, 1845–1851 (2009).
Lermite, E. et al. Risk factors of pancreatic fistula and delayed gastric emptying after pancreaticoduodenectomy with pancreaticogastrostomy. J. Am. Coll. Surg. 204, 588–596 (2007).
Fleming, J. B. et al. Influence of obesity on cancer-related outcomes after pancreatectomy to treat pancreatic adenocarcinoma. Arch. Surg. 144, 216–221 (2009).
Tsai, S. et al. Impact of obesity on perioperative outcomes and survival following pancreaticoduodenectomy for pancreatic cancer: a large single-institution study. J. Gastrointest. Surg. 14, 1143–1150 (2010).
de Vries, E. et al. Lifestyle changes and reduction of colon cancer incidence in Europe: a scenario study of physical activity promotion and weight reduction. Eur. J. Cancer 46, 2605–2616 (2010).
Acknowledgements
Work on the link between obesity, weight loss and cancer risk in the authors' laboratory is funded by the Medical Research Council (UK) Medical Research Foundation.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Box 1
Measurement of excess body weight (DOC 33 kb)
Rights and permissions
About this article
Cite this article
Kant, P., Hull, M. Excess body weight and obesity—the link with gastrointestinal and hepatobiliary cancer. Nat Rev Gastroenterol Hepatol 8, 224–238 (2011). https://doi.org/10.1038/nrgastro.2011.23
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2011.23
This article is cited by
-
Exercise suppresses tumor growth independent of high fat food intake and associated immune dysfunction
Scientific Reports (2022)
-
Baseline liver steatosis has no impact on liver metastases and overall survival in rectal cancer patients
BMC Cancer (2021)
-
Evidence from big data in obesity research: international case studies
International Journal of Obesity (2020)
-
Adiposity and gastrointestinal cancers: epidemiology, mechanisms and future directions
Nature Reviews Gastroenterology & Hepatology (2018)
-
Mechanistic and therapeutic advances in non-alcoholic fatty liver disease by targeting the gut microbiota
Frontiers of Medicine (2018)