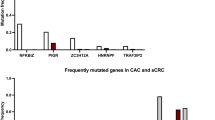
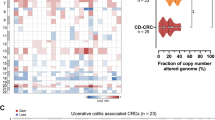
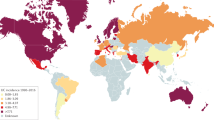
Abstract
One of the most serious complications of ulcerative colitis is the development of colorectal cancer. Screening patients with ulcerative colitis by standard histological examination of random intestinal biopsy samples might be inefficient as a method of cancer surveillance. This Review focuses on the current understanding of the pathogenesis of ulcerative colitis-associated colorectal cancer and how this knowledge can be transferred into patient management to assist clinicians and pathologists in identifying patients with ulcerative colitis who have an increased risk of colorectal cancer. Inflammation-driven mechanisms of DNA damage, including the generation and effects of reactive oxygen species, microsatellite instability, telomere shortening and chromosomal instability, are reviewed, as are the molecular responses to genomic stress. We also discuss how these mechanisms can be translated into usable biomarkers. Although progress has been made in the understanding of inflammation-driven carcinogenesis, markers based on these findings possess insufficient sensitivity or specificity to be usable as reliable biomarkers for risk of colorectal cancer development in patients with ulcerative colitis. However, screening for mutations in p53 could be relevant in the surveillance of patients with ulcerative colitis. Several other new biomarkers, including senescence markers and α-methylacyl-CoA-racemase, might be future candidates for preneoplastic markers in ulcerative colitis.
Key Points
-
Pathogenesis of colorectal cancer in patients with ulcerative colitis is driven by inflammation-dependent mechanisms
-
The generation of reactive oxygen species in epithelial cells and the exposure of epithelial cell DNA to these molecules may be important for the development of colorectal cancer in ulcerative colitis
-
p53 mutations and chromosomal instability are the most promising biomarkers of premalignancy, and could be used in combination with histological evaluation to identify patients at high risk of developing colorectal cancer
-
Senescence and α-methylacyl-CoA-racemase are promising new candidates for preneoplastic biomarkers in ulcerative colitis
-
The clinical implications and reliability of biomarkers of premalignancy in ulcerative colitis remains to be established in prospective studies
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Winther, K. V., Jess, T., Langholz, E., Munkholm, P. & Binder, V. Survival and cause-specific mortality in ulcerative colitis: follow-up of a population-based cohort in Copenhagen County. Gastroenterology 125, 1576–1582 (2003).
Baumgart, D. C. & Sandborn, W. J. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 369, 1641–1657 (2007).
Eaden, J. A., Abrams, K. R. & Mayberry, J. F. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 48, 526–535 (2001).
National Cancer Institute. Surveillance Epidemiology and End Results (SEER) [online], (2010).
Riddell, R. H. et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum. Pathol. 14, 931–968 (1983).
Itzkowitz, S. H. & Yio, X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G7–G17 (2004).
Rutter, M. et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 126, 451–459 (2004).
Abraham, C. & Cho, J. H. Inflammatory bowel disease. N. Engl. J. Med. 361, 2066–2078 (2009).
Cario, E. Toll-like receptors in inflammatory bowel diseases: a decade later. Inflamm. Bowel Dis. 16, 1583–1597 (2010).
Ogier-Denis, E., Mkaddem, S. B. & Vandewalle, A. NOX enzymes and Toll-like receptor signaling. Semin. Immunopathol. 30, 291–300 (2008).
Roessner, A., Kuester, D., Malfertheiner, P. & Schneider-Stock, R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol. Res. Pract. 204, 511–524 (2008).
Rosen, G. M., Pou, S., Ramos, C. L., Cohen, M. S. & Britigan, B. E. Free radicals and phagocytic cells. FASEB J. 9, 200–209 (1995).
Jackson, A. L. & Loeb, L. A. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat. Res. 477, 7–21 (2001).
Grisham, M. B. Oxidants and free radicals in inflammatory bowel disease. Lancet 344, 859–861 (1994).
Sawa, T. & Ohshima, H. Nitrative DNA damage in inflammation and its possible role in carcinogenesis. Nitric Oxide 14, 91–100 (2006).
Caulfield, J. L., Wishnok, J. S. & Tannenbaum, S. R. Nitric oxide-induced deamination of cytosine and guanine in deoxynucleosides and oligonucleotides. J. Biol. Chem. 273, 12689–12695 (1998).
Sawa, T., Akaike, T. & Maeda, H. Tyrosine nitration by peroxynitrite formed from nitric oxide and superoxide generated by xanthine oxidase. J. Biol. Chem. 275, 32467–32474 (2000).
Kennedy, L. J., Moore, K. Jr, Caulfield, J. L., Tannenbaum, S. R. & Dedon, P. C. Quantitation of 8-oxoguanine and strand breaks produced by four oxidizing agents. Chem. Res. Toxicol. 10, 386–392 (1997).
Salgo, M. G., Bermúdez, E., Squadrito, G. L. & Pryor, W. A. Peroxynitrite causes DNA damage and oxidation of thiols in rat thymocytes [corrected]. Arch. Biochem. Biophys. 322, 500–505 (1995).
Juedes, M. J. & Wogan, G. N. Peroxynitrite-induced mutation spectra of pSP189 following replication in bacteria and in human cells. Mutat. Res. 349, 51–61 (1996).
Marnett, L. J. Oxy radicals, lipid peroxidation and DNA damage. Toxicology 181–182, 219–222 (2002).
Simmonds, N. J. et al. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology 103, 186–196 (1992).
Keshavarzian, A. et al. Excessive production of reactive oxygen metabolites by inflamed colon: analysis by chemiluminescence probe. Gastroenterology 103, 177–185 (1992).
Kruidenier, L., Kuiper, I., Lamers, C. B. & Verspaget, H. W. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J. Pathol. 201, 28–36 (2003).
Andresen, L. et al. Activation of nuclear factor κB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut 54, 503–509 (2005).
Seidelin, J. B. & Nielsen, O. H. Continuous cytokine exposure of colonic epithelial cells induces DNA damage. Eur. J. Gastroenterol. Hepatol. 17, 363–369 (2005).
Kimura, H. et al. Increased expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in colonic mucosa of patients with active ulcerative colitis. Gut 42, 180–187 (1998).
Hussain, S. P., Hofseth, L. J. & Harris, C. C. Radical causes of cancer. Nat. Rev. Cancer 3, 276–285 (2003).
Sánchez-Alcázar, J. A. et al. Tumor necrosis factor-α increases the steady-state reduction of cytochrome b of the mitochondrial respiratory chain in metabolically inhibited L929 cells. J. Biol. Chem. 275, 13353–13361 (2000).
Schulze-Osthoff, K. et al. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J. Biol. Chem. 267, 5317–5323 (1992).
Yan, B. et al. Tumor necrosis factor-α is a potent endogenous mutagen that promotes cellular transformation. Cancer Res. 66, 11565–11570 (2006).
Holmes, E. W., Yong, S. L., Eiznhamer, D. & Keshavarzian, A. Glutathione content of colonic mucosa: evidence for oxidative damage in active ulcerative colitis. Dig. Dis. Sci. 43, 1088–1095 (1998).
Koutroubakis, I. E. et al. Decreased total and corrected antioxidant capacity in patients with inflammatory bowel disease. Dig. Dis. Sci. 49, 1433–1437 (2004).
Wu, W. S. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 25, 695–705 (2006).
Svineng, G., Ravuri, C., Rikardsen, O., Huseby, N. E. & Winberg, J. O. The role of reactive oxygen species in integrin and matrix metalloproteinase expression and function. Connect. Tissue Res. 49, 197–202 (2008).
Itzkowitz, S. H., Present, D. H. & Crohn's and Colitis Foundation of America Colon Cancer in IBD Study Group. Consensus conference: colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm. Bowel Dis. 11, 314–321 (2005).
Chambers, W. M., Warren, B. F., Jewell, D. P. & Mortensen, N. J. Cancer surveillance in ulcerative colitis. Br. J. Surg. 92, 928–936 (2005).
Fujii, S., Katsumata, D. & Fujimori, T. Limits of diagnosis and molecular markers for early detection of ulcerative colitis-associated colorectal neoplasia. Digestion 77 (Suppl. 1), 2–12 (2008).
Melville, D. M. et al. Observer study of the grading of dysplasia in ulcerative colitis: comparison with clinical outcome. Hum. Pathol. 20, 1008–1014 (1989).
Collins, P. D., Mpofu, C., Watson, A. J. & Rhodes, J. M. Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease. Cochrane Database of Systematic Reviews, Issue 2. Art. No.: CD000279. doi:10.1002/14651858.CD000279.pub3 (2006).
Brentnall, T. A. et al. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology 107, 369–378 (1994).
Leedham, S. J. et al. Clonality, founder mutations, and field cancerization in human ulcerative colitis-associated neoplasia. Gastroenterology 136, 542–550 (2009).
Powell, S. M. et al. APC mutations occur early during colorectal tumorigenesis. Nature 359, 235–237 (1992).
Holzmann, K. et al. Comparative analysis of histology, DNA content, p53 and Ki-ras mutations in colectomy specimens with long-standing ulcerative colitis. Int. J. Cancer 76, 1–6 (1998).
Hussain, S. P. et al. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res. 60, 3333–3337 (2000).
Lashner, B. A., Shapiro, B. D., Husain, A. & Goldblum, J. R. Evaluation of the usefulness of testing for p53 mutations in colorectal cancer surveillance for ulcerative colitis. Am. J. Gastroenterol. 94, 456–462 (1999).
Ambs, S. et al. Relationship between p53 mutations and inducible nitric oxide synthase expression in human colorectal cancer. J. Natl Cancer Inst. 91, 86–88 (1999).
Fujii, S., Fujimori, T. & Chiba, T. Usefulness of analysis of p53 alteration and observation of surface microstructure for diagnosis of ulcerative colitis-associated colorectal neoplasia. J. Exp. Clin. Cancer Res. 22, 107–115 (2003).
Finlay, C. A. et al. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol. Cell Biol. 8, 531–539 (1988).
Vousden, K. H. Activation of the p53 tumor suppressor protein. Biochim. Biophys. Acta 1602, 47–59 (2002).
Brooks, C. L. & Gu, W. Dynamics in the p53–Mdm2 ubiquitination pathway. Cell Cycle 3, 895–899 (2004).
Seril, D. N., Liao, J., Yang, G. Y. & Yang, C. S. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis 24, 353–362 (2003).
Willenbucher, R. F., Zelman, S. J., Ferrell, L. D., Moore, D. H. 2nd & Waldman, F. M. Chromosomal alterations in ulcerative colitis-related neoplastic progression. Gastroenterology 113, 791–801 (1997).
Aust, D. E. et al. Chromosomal alterations in ulcerative colitis-related and sporadic colorectal cancers by comparative genomic hybridization. Hum. Pathol. 31, 109–114 (2000).
Willenbucher, R. F. et al. Genomic instability is an early event during the progression pathway of ulcerative-colitis-related neoplasia. Am. J. Pathol. 154, 1825–1830 (1999).
Chen, R. et al. DNA fingerprinting abnormalities can distinguish ulcerative colitis patients with dysplasia and cancer from those who are dysplasia/cancer-free. Am. J. Pathol. 162, 665–672 (2003).
Rabinovitch, P. S. et al. Pancolonic chromosomal instability precedes dysplasia and cancer in ulcerative colitis. Cancer Res. 59, 5148–5153 (1999).
Bronner, M. P. et al. Genomic biomarkers to improve ulcerative colitis neoplasia surveillance. Am. J. Pathol. 173, 1853–1860 (2008).
Jackson, S. P. Sensing and repairing DNA double-strand breaks. Carcinogenesis 23, 687–696 (2002).
Karanjawala, Z. E., Murphy, N., Hinton, D. R., Hsieh, C. L. & Lieber, M. R. Oxygen metabolism causes chromosome breaks and is associated with the neuronal apoptosis observed in DNA double-strand break repair mutants. Curr. Biol. 12, 397–402 (2002).
Dickey, J. S. et al. H2AX: functional roles and potential applications. Chromosoma 118, 683–692 (2009).
Fernandez-Capetillo, O., Lee, A., Nussenzweig, M. & Nussenzweig, A. H2AX: the histone guardian of the genome. DNA Repair (Amst.) 3, 959–967 (2004).
Vilenchik, M. M. & Knudson, A. G. Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proc. Natl Acad. Sci. USA 100, 12871–12876 (2003).
Cahill, D., Connor, B. & Carney, J. P. Mechanisms of eukaryotic DNA double strand break repair. Front. Biosci. 11, 1958–1976 (2006).
Rogakou, E. P., Boon, C., Redon, C. & Bonner, W. M. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 146, 905–916 (1999).
Nuciforo, P. G., Luise, C., Capra, M., Pelosi, G. & d'Adda di Fagagna, F. Complex engagement of DNA damage response pathways in human cancer and in lung tumor progression. Carcinogenesis 28, 2082–2088 (2007).
Blanco, D. et al. Molecular analysis of a multistep lung cancer model induced by chronic inflammation reveals epigenetic regulation of p16 and activation of the DNA damage response pathway. Neoplasia 9, 840–852 (2007).
Tanaka, T. et al. Cytometry of ATM activation and histone H2AX phosphorylation to estimate extent of DNA damage induced by exogenous agents. Cytometry A 71, 648–661 (2007).
Risques, R. A. et al. Ulcerative colitis-associated colorectal cancer arises in a field of short telomeres, senescence, and inflammation. Cancer Res. 71, 1669–1679 (2011).
Risques, R. A. et al. Ulcerative colitis is a disease of accelerated colon aging: evidence from telomere attrition and DNA damage. Gastroenterology 135, 410–418 (2008).
O'Sullivan, J. N. et al. Chromosomal instability in ulcerative colitis is related to telomere shortening. Nat. Genet. 32, 280–284 (2002).
von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344 (2002).
d'Adda di Fagagna, F. et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 (2003).
Ben-Porath, I. & Weinberg, R. A. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 37, 961–976 (2005).
Collado, M. & Serrano, M. The power and the promise of oncogene-induced senescence markers. Nat. Rev. Cancer 6, 472–476 (2006).
Coppé, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Wang, D., Kreutzer, D. A. & Essigmann, J. M. Mutagenicity and repair of oxidative DNA damage: insights from studies using defined lesions. Mutat. Res. 400, 99–115 (1998).
Valavanidis, A., Vlachogianni, T. & Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 27, 120–139 (2009).
Kamiya, H. et al. 8-hydroxyguanine (7,8-dihydro-8-oxoguanine) in hot spots of the c-Ha-ras gene: effects of sequence contexts on mutation spectra. Carcinogenesis 16, 883–889 (1995).
Klein, J. C. et al. Repair and replication of plasmids with site-specific 8-oxodG and 8-AAFdG residues in normal and repair-deficient human cells. Nucleic Acids Res. 20, 4437–4443 (1992).
D'Incà, R. et al. Oxidative DNA damage in the mucosa of ulcerative colitis increases with disease duration and dysplasia. Inflamm. Bowel Dis. 10, 23–27 (2004).
Gushima, M. et al. Altered expression of MUTYH and an increase in 8-hydroxydeoxyguanosine are early events in ulcerative colitis-associated carcinogenesis. J. Pathol. 219, 77–86 (2009).
European Standards Committee on Oxidative DNA Damage (ESCODD). Measurement of DNA oxidation in human cells by chromatographic and enzymic methods. Free Radic. Biol. Med. 34, 1089–1099 (2003).
ESCODD (European Standards Committee on Oxidative DNA Damage). Comparative analysis of baseline 8-oxo-7, 8-dihydroguanine in mammalian cell DNA, by different methods in different laboratories: an approach to consensus. Carcinogenesis 23, 2129–2133 (2002).
Rodriguez, H., Jurado, J., Laval, J. & Dizdaroglu, M. Comparison of the levels of 8-hydroxyguanine in DNA as measured by gas chromatography mass spectrometry following hydrolysis of DNA by Escherichia coli Fpg protein or formic acid. Nucleic Acids Res. 28, E75 (2000).
Löfberg, R., Broström, O., Karlén, P., Ost, A. & Tribukait, B. DNA aneuploidy in ulcerative colitis: reproducibility, topographic distribution, and relation to dysplasia. Gastroenterology 102, 1149–1154 (1992).
Rubin, C. E. et al. DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology 103, 1611–1620 (1992).
Rutegård, J., Ahsgren, L., Stenling, R. & Roos, G. DNA content and mucosal dysplasia in ulcerative colitis. Flow cytometric analysis in patients with dysplastic or indefinite morphologic changes in the colorectal mucosa. Dis. Colon Rectum 32, 1055–1059 (1989).
Befrits, R., Hammarberg, C., Rubio, C., Jaramillo, E. & Tribukait, B. DNA aneuploidy and histologic dysplasia in long-standing ulcerative colitis. A 10-year follow-up study. Dis. Colon Rectum 37, 313–319 (1994).
Risques, R. A., Rabinovitch, P. S. & Brentnall, T. A. Cancer surveillance in inflammatory bowel disease: new molecular approaches. Curr. Opin. Gastroenterol. 22, 382–390 (2006).
Stenling, R., Jonsson, B. O., Palmqvist, R. & Rutegård, J. N. DNA aneuploidy in ulcerative colitis and in colorectal carcinoma—a comparative study. Anal. Cell Pathol. 18, 69–72 (1999).
Thibodeau, S. N., Bren, G. & Schaid, D. Microsatellite instability in cancer of the proximal colon. Science 260, 816–819 (1993).
Rhyu, M. G., Park, W. S. & Meltzer, S. J. Microsatellite instability occurs frequently in human gastric carcinoma. Oncogene 9, 29–32 (1994).
Fleisher, A. S. et al. Microsatellite instability in inflammatory bowel disease-associated neoplastic lesions is associated with hypermethylation and diminished expression of the DNA mismatch repair gene, hMLH1. Cancer Res. 60, 4864–4868 (2000).
Brentnall, T. A. et al. Microsatellite instability in nonneoplastic mucosa from patients with chronic ulcerative colitis. Cancer Res. 56, 1237–1240 (1996).
Fujiwara, I., Yashiro, M., Kubo, N., Maeda, K. & Hirakawa, K. Ulcerative colitis-associated colorectal cancer is frequently associated with the microsatellite instability pathway. Dis. Colon Rectum 51, 1387–1394 (2008).
Løvig, T., Andersen, S. N., Clausen, O. P. & Rognum, T. O. Microsatellite instability in long-standing ulcerative colitis. Scand. J. Gastroenterol. 42, 586–591 (2007).
Tahara, T. et al. Clinical significance of microsatellite instability in the inflamed mucosa for the prediction of colonic neoplasms in patients with ulcerative colitis. J. Gastroenterol. Hepatol. 20, 710–715 (2005).
Boland, C. R. et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 58, 5248–5257 (1998).
Ritz-Timme, S. & Collins, M. J. Racemization of aspartic acid in human proteins. Ageing Res. Rev. 1, 43–59 (2002).
Went, P. T., Sauter, G., Oberholzer, M. & Bubendorf, L. Abundant expression of AMACR in many distinct tumour types. Pathology 38, 426–432 (2006).
Tosoian, J. & Loeb, S. PSA and beyond: the past, present, and future of investigative biomarkers for prostate cancer. ScientificWorldJournal 10, 1919–1931 (2010).
Dorer, R. & Odze, R. D. AMACR immunostaining is useful in detecting dysplastic epithelium in Barrett's esophagus, ulcerative colitis, and Crohn's disease. Am. J. Surg. Pathol. 30, 871–877 (2006).
Gerdes, J. et al. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am. J. Pathol. 138, 867–873 (1991).
Andersen, S. N., Rognum, T. O., Bakka, A. & Clausen, O. P. Ki-67: a useful marker for the evaluation of dysplasia in ulcerative colitis. Mol. Pathol. 51, 327–332 (1998).
Wong, N. A., Mayer, N. J., MacKell, S., Gilmour, H. M. & Harrison, D. J. Immunohistochemical assessment of Ki67 and p53 expression assists the diagnosis and grading of ulcerative colitis-related dysplasia. Histopathology 37, 108–114 (2000).
Noffsinger, A. E., Miller, M. A., Cusi, M. V. & Fenoglio-Preiser, C. M. The pattern of cell proliferation in neoplastic and nonneoplastic lesions of ulcerative colitis. Cancer 78, 2307–2312 (1996).
Bartsch, H. & Nair, J. Accumulation of lipid peroxidation-derived DNA lesions: potential lead markers for chemoprevention of inflammation-driven malignancies. Mutat. Res. 591, 34–44 (2005).
Nair, J. et al. Increased etheno-DNA adducts in affected tissues of patients suffering from Crohn's disease, ulcerative colitis, and chronic pancreatitis. Antioxid. Redox Signal. 8, 1003–1010 (2006).
van Staa, T. P., Card, T., Logan, R. F. & Leufkens, H. G. 5-Aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: a large epidemiological study. Gut 54, 1573–1578 (2005).
Velayos, F. S., Terdiman, J. P. & Walsh, J. M. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am. J. Gastroenterol. 100, 1345–1353 (2005).
Clapper, M. L. et al. 5-aminosalicylic acid inhibits colitis-associated colorectal dysplasias in the mouse model of azoxymethane/dextran sulfate sodium-induced colitis. Inflamm. Bowel Dis. 14, 1341–1347 (2008).
Ahnfelt-Rønne, I. et al. Clinical evidence supporting the radical scavenger mechanism of 5-aminosalicylic acid. Gastroenterology 98, 1162–1169 (1990).
Nielsen, O. H. In vitro studies on the significance of arachidonate metabolism and other oxidative processes in the inflammatory response of human neutrophils and macrophages. With special reference to chronic inflammatory bowel disease. Scand. J. Gastroenterol. Suppl. 150, 1–21 (1988).
Nielsen, O. H. & Munck, L. K. Drug insight: aminosalicylates for the treatment of IBD. Nat. Clin. Pract Gastroenterol. Hepatol. 4, 160–170 (2007).
Yppolito, R. et al. On the antioxidant properties of therapeutic drugs: quenching of singlet molecular oxygen by aminosalicylic acids. Redox Rep. 7, 229–233 (2002).
Simmonds, N. J., Millar, A. D., Blake, D. R. & Rampton, D. S. Antioxidant effects of aminosalicylates and potential new drugs for inflammatory bowel disease: assessment in cell-free systems and inflamed human colorectal biopsies. Aliment. Pharmacol. Ther. 13, 363–372 (1999).
Luciani, M. G., Campregher, C., Fortune, J. M., Kunkel, T. A. & Gasche, C. 5-ASA affects cell cycle progression in colorectal cells by reversibly activating a replication checkpoint. Gastroenterology 132, 221–235 (2007).
Stolfi, C. et al. Cyclooxygenase-2-dependent and -independent inhibition of proliferation of colon cancer cells by 5-aminosalicylic acid. Biochem. Pharmacol. 75, 668–676 (2008).
Koelink, P. J. et al. 5-aminosalicylic acid interferes in the cell cycle of colorectal cancer cells and induces cell death modes. Inflamm. Bowel Dis. 16, 379–389 (2010).
Speckmann, B. et al. Proinflammatory cytokines down-regulate intestinal selenoprotein P biosynthesis via NOS2 induction. Free Radic. Biol. Med. 49, 777–785 (2010).
Linehan, J. D., Kolios, G., Valatas, V., Robertson, D. A. & Westwick, J. Immunomodulatory cytokines suppress epithelial nitric oxide production in inflammatory bowel disease by acting on mononuclear cells. Free Radic. Biol. Med. 39, 1560–1569 (2005).
Potoka, D. A. et al. Inhibition of NF-κB by IκB prevents cytokine-induced NO production and promotes enterocyte apoptosis in vitro. Shock 14, 366–373 (2000).
Acknowledgements
This study was supported by grants from Fonden til Lægevidenskabens Fremme (the A. P. Møller Foundation), the Family Erichsen Memorial Foundation, the Lundbeck Foundation, the Axel Muusfeldts Foundation, and the Aase and Ejnar Danielsen Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Thorsteinsdottir, S., Gudjonsson, T., Nielsen, O. et al. Pathogenesis and biomarkers of carcinogenesis in ulcerative colitis. Nat Rev Gastroenterol Hepatol 8, 395–404 (2011). https://doi.org/10.1038/nrgastro.2011.96
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2011.96
This article is cited by
-
Unlocking the Anticancer Potential of Frankincense Essential Oils (FEOs) Through Nanotechnology: A Review
Molecular Biotechnology (2023)
-
Zinc and Selenium in Inflammatory Bowel Disease: Trace Elements with Key Roles?
Biological Trace Element Research (2021)
-
Telomere dysfunction activates YAP1 to drive tissue inflammation
Nature Communications (2020)
-
Clonal evolution of colorectal cancer in IBD
Nature Reviews Gastroenterology & Hepatology (2017)
-
Comparative analysis of copy number variations in ulcerative colitis associated and sporadic colorectal neoplasia
BMC Cancer (2016)