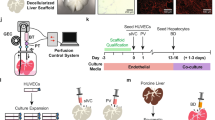
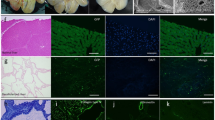
Abstract
Initially hailed as the ultimate solution to organ failure, engineering of vascularized tissues such as the liver has stalled because of the need for a well-structured circulatory system that can maintain the cells seeded inside the construct. A new approach has evolved to overcome this obstacle. Whole-organ decellularization is a method that retains most of the native vascular structures of the organ, providing microcirculatory support and structure, which can be anastomosed with the recipient circulation. The technique was first applied to the heart and then adapted for the liver. Several studies have shown that cells can be eliminated, the extracellular matrix and vasculature are reasonably preserved and, after repopulation with hepatocytes, these grafts can perform hepatic functions in vitro and in vivo. Progress is rapidly being made as researchers are addressing several key challenges to whole-organ tissue engineering, such as ensuring correct cell distribution, nonparenchymal cell seeding, blood compatibility, immunological concerns, and the source of cells and matrices.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Punch, J. D., Hayes, D. H., LaPorte, F. B., McBride, V. & Seely, M. S. Organ donation and utilization in the United States, 1996–2005. Am. J. Transplant. 7, 1327–1338 (2007).
Burg, T., Cass, C. A. P., Groff, R., Pepper, M. & Burg, K. J. L. Building off-the-shelf tissue-engineered composites. Philos. Transact. A: Math. Phys. Eng. Sci. 368, 1839–1862 (2010).
Lysaght, M. J., Jaklenec, A. & Deweerd, E. Great expectations: private sector activity in tissue engineering, regenerative medicine, and stem cell therapeutics. Tissue Eng. Part A 14, 305–315 (2008).
Fox, I. J. et al. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N. Engl. J. Med. 338, 1422–1426 (1998).
Smets, F., Najimi, M. & Sokal, E. M. Cell transplantation in the treatment of liver diseases. Pediatr. Transplant. 12, 6–13 (2008).
Mei, J. et al. Improved survival of fulminant liver failure by transplantation of microencapsulated cryopreserved porcine hepatocytes in mice. Cell Transplant. 18, 101–110 (2009).
Navarro-Alvarez, N. et al. Intramuscular transplantation of engineered hepatic tissue constructs corrects acute and chronic liver failure in mice. J. Hepatol. 52, 211–219 (2010).
Mooney, D. J. & Vandenburgh, H. Cell delivery mechanisms for tissue repair. Cell Stem Cell 2, 205–213 (2008).
Chung, S. & King, M. W. Design concepts and strategies for tissue engineering scaffolds. Biotechnol. Appl. Biochem. 58, 423–438 (2011).
Ohashi, K. et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat. Med. 13, 880–885 (2007).
Soto-Gutierrez, A. et al. Construction and transplantation of an engineered hepatic tissue using a polyaminourethane-coated nonwoven polytetrafluoroethylene fabric. Transplantation 83, 129–137 (2007).
Kulig, K. M. & Vacanti, J. P. Hepatic tissue engineering. Transpl. Immunol. 12, 303–310 (2004).
Carraro, A. et al. In vitro analysis of a hepatic device with intrinsic microvascular-based channels. Biomed. Microdevices 10, 795–805 (2008).
Yokoyama, T. et al. In vivo engineering of metabolically active hepatic tissues in a neovascularized subcutaneous cavity. Am. J. Transplant. 6, 50–59 (2006).
Kedem, A. et al. Vascular endothelial growth factor-releasing scaffolds enhance vascularization and engraftment of hepatocytes transplanted on liver lobes. Tissue Eng. 11, 715–722 (2005).
Hou, Y. T., Ijima, H., Takei, T. & Kawakami, K. Growth factor/heparin-immobilized collagen gel system enhances viability of transplanted hepatocytes and induces angiogenesis. J. Biosci. Bioeng. 112, 265–272 (2011).
Uyama, S., Kaufmann, P. M., Takeda, T. & Vacanti, J. P. Delivery of whole liver-equivalent hepatocyte mass using polymer devices and hepatotrophic stimulation. Transplantation 55, 932–935 (1993).
Lutolf, M. P. & Hubbell, J. A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 23, 47–55 (2005).
Badylak, S. F. The extracellular matrix as a biologic scaffold material. Biomaterials 28, 3587–3593 (2007).
Badylak, S. F., Taylor, D. & Uygun, K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 13, 27–53 (2011).
Yoo, J. J., Meng, J., Oberpenning, F. & Atala, A. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology 51, 221–225 (1998).
Schechner, J. S. et al. Engraftment of a vascularized human skin equivalent. FASEB J. 17, 2250–2256 (2003).
Macchiarini, P. et al. Clinical transplantation of a tissue-engineered airway. Lancet 372, 2023–2030 (2008).
Ott, H. C. et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat. Med. 14, 213–221 (2008).
Uygun, B. E. et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 16, 814–820 (2010).
Bao, J. et al. Construction of a portal implantable functional tissue-engineered liver using perfusion-decellularized matrix and hepatocytes in rats. Cell Transplant. 20, 753–766 (2011).
Baptista, P. M. et al. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 53, 604–617 (2011).
Soto-Gutierrez, A. et al. A whole-organ regenerative medicine approach for liver replacement. Tissue Eng. Part C Methods 17, 677–686 (2011).
Zhou, P. et al. Decellularized liver matrix as a carrier for the transplantation of human fetal and primary hepatocytes in mice. Liver Transpl. 17, 418–427 (2011).
Barakat, O. et al. Use of decellularized porcine liver for engineering humanized liver organ. J. Surg. Res. 173, e11–e25 (2012).
Ott, H. C. et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 16, 927–933 (2010).
Petersen, T. H. et al. Tissue-engineered lungs for in vivo implantation. Science 329, 538–541 (2010).
Price, A. P., England, K. A., Matson, A. M., Blazar, B. R. & Panoskaltsis-Mortari, A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng. Part A 16, 2581–2591 (2010).
Song, J. J. & Ott, H. C. Organ engineering based on decellularized matrix scaffolds. Trends Mol. Med. 17, 424–432 (2011).
Amenta, P. S. & Harrison, D. Expression and potential role of the extracellular matrix in hepatic ontogenesis: a review. Microsc. Res. Tech. 39, 372–386 (1997).
Dunn, J. C., Tompkins, R. G. & Yarmush, M. L. Hepatocytes in collagen sandwich: evidence for transcriptional and translational regulation. J. Cell Biol. 116, 1043–1053 (1992).
Sellaro, T. L. et al. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng. Part A 16, 1075–1082 (2010).
Ishii, T. et al. Effects of extracellular matrixes and growth factors on the hepatic differentiation of human embryonic stem cells. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G313–G321 (2008).
Kim, S. H., Turnbull, J. & Guimond, S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 209, 139–151 (2011).
Wang, Y. et al. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology 53, 293–305 (2011).
Marongiu, F. et al. Hepatic differentiation of amniotic epithelial cells. Hepatology 53, 1719–1729 (2011).
Crapo, P. M., Gilbert, T. W. & Badylak, S. F. An overview of tissue and whole-organ decellularization processes. Biomaterials 32, 3233–3243 (2011).
Horbett, T. A., Ratner, B. D., Schakenraad, J. M. & Schoen, F. J. in Biomaterials Science: an Introduction to Materials in Medicine (eds Ratner, B. D., Hoffman, A. S., Schoen, F. J. & Lemons, J. E.) 147–164 (Academic Press, San Diego, CA, 1996).
Uygun, B. E. et al. Decellularization and recellularization of whole livers. J. Vis. Exp. e2394 (2011).
Yagi, H. et al. Human-scale whole-organ. bioengineering for liver transplantation: a regenerative medicine approach. Cell Transplant (in press).
Ohi, Y. et al. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nat. Cell Biol. 13, 541–549 (2011).
Kim, K. et al. Epigenetic memory in induced pluripotent stem cells. Nature 467, 285–290 (2010).
Polo, J. M. et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat. Biotechnol. 28, 848–855 (2010).
Kakinuma, S., Nakauchi, H. & Watanabe, M. Hepatic stem/progenitor cells and stem-cell transplantation for the treatment of liver disease. J. Gastroenterol. 44, 167–172 (2009).
Izamis, M. L. et al. Better than fresh: simple ex vivo perfusion optimizes ischemic and fresh donor livers for transplantation and significantly enhanced hepatocyte yields. Hepatology 54, 688A–688A (2011).
Oertel, M. et al. Purification of fetal liver stem/progenitor cells containing all the repopulation potential for normal adult rat liver. Gastroenterology 134, 823–832 (2008).
Galili, U. Induced anti-non gal antibodies in human xenograft recipients. Transplantation 93, 11–16 (2012).
Daly, K. A. et al. Effect of the alphaGal epitope on the response to small intestinal submucosa extracellular matrix in a nonhuman primate model. Tissue Eng. Part A 15, 3877–3888 (2009).
Xu, H. et al. A porcine-derived acellular dermal scaffold that supports soft tissue regeneration: removal of terminal galactose-alpha-(1,3)-galactose and retention of matrix structure. Tissue Eng. Part A 15, 1807–1819 (2009).
Abt, P. L., Fisher, C. A. & Singhal, A. K. Donation after cardiac death in the US: history and use. J. Am. Coll. Surg. 203, 208–225 (2006).
Tolboom, H. et al. Recovery of warm ischemic rat liver grafts by normothermic extracorporeal perfusion. Transplantation 87, 170–177 (2009).
Coller, B. S., Beer, J. H., Scudder, L. E. & Steinberg, M. H. Collagen-platelet interactions: evidence for a direct interaction of collagen with platelet GPIa/IIa and an indirect interaction with platelet GPIIb/IIIa mediated by adhesive proteins. Blood 74, 182–192 (1989).
Bao, J. et al. Construction of a portal implantable functional tissue-engineered liver using perfusion-decellularized matrix and hepatocytes in rats. Cell Transplant. 20, 753–766 (2011).
Delrivière, L. et al. Detailed modified technique for safer harvesting and preparation of liver graft in the rat. Microsurgery 17, 690–696 (1996).
Delrivière, L. et al. Technical details for safer venous and biliary anastomoses for liver transplantation in the rat. Microsurgery 18, 12–18 (1998).
Nahmias, Y., Berthiaume, F. & Yarmush, M. L. Integration of technologies for hepatic tissue engineering. Adv. Biochem. Eng. Biotechnol. 103, 309–329 (2007).
Strauss, K. A. et al. Management of hyperbilirubinemia and prevention of kernicterus in 20 patients with Crigler-Najjar disease. Eur. J. Pediatr. 165, 306–319 (2006).
van der Veere, C. N. et al. Current therapy for Crigler-Najjar syndrome type 1: report of a world registry. Hepatology 24, 311–315 (1996).
Bernal, W., Auzinger, G., Dhawan, A. & Wendon, J. Acute liver failure. Lancet 376, 190–201 (2010).
Bower, W. A., Johns, M., Margolis, H. S., Williams, I. T. & Bell, B. P. Population-based surveillance for acute liver failure. Am. J. Gastroenterol. 102, 2459–2463 (2007).
Acknowledgements
Funding from the National Institutes of Health (R01DK084053, R01DK096075R01, K99DK088962), National Science Foundation (CBET 0853569) and the Shriners Hospitals for Children are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
B. E. Uygun, M. L. Yarmush and K. Uygun are patent applicants for Massachusetts General Hospital, Boston, MA, USA. The patent application relates to hepatic tissue engineering processes (application number WO2011002926).
Rights and permissions
About this article
Cite this article
Uygun, B., Yarmush, M. & Uygun, K. Application of whole-organ tissue engineering in hepatology. Nat Rev Gastroenterol Hepatol 9, 738–744 (2012). https://doi.org/10.1038/nrgastro.2012.140
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2012.140
This article is cited by
-
Three Dimensional Bioprinting for Hepatic Tissue Engineering: From In Vitro Models to Clinical Applications
Tissue Engineering and Regenerative Medicine (2024)
-
Gelatin Promotes Cell Retention Within Decellularized Heart Extracellular Matrix Vasculature and Parenchyma
Cellular and Molecular Bioengineering (2020)
-
Processing of collagen based biomaterials and the resulting materials properties
BioMedical Engineering OnLine (2019)
-
Impact of Percoll purification on isolation of primary human hepatocytes
Scientific Reports (2019)
-
„Liver engineering“ als neue Quelle von Spenderorganen
Der Chirurg (2016)