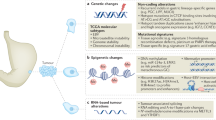
Key Points
-
Globally, gastric cancer is highly prevalent and accounts for a considerable amount of mortality around the world, with poor survival rates
-
Understanding the genetic basis of gastric cancer will offer insights into its pathogenesis, as well as the possibility of identifying new biomarkers and novel treatment targets
-
An inherited component is present in <3% of cases of gastric cancer
-
Most genetic changes associated with gastric cancer are acquired and are a result of chromosomal instability, microsatellite instability, changes in microRNA profile, somatic gene mutations or functional single nucleotide polymorphisms
Abstract
Gastric cancer remains highly prevalent and accounts for a notable proportion of global cancer mortality. This cancer is also associated with poor survival rates. Understanding the genetic basis of gastric cancer will offer insights into its pathogenesis, help identify new biomarkers and novel treatment targets, aid prognostication and could be central to developing individualized treatment strategies in the future. An inherited component contributes to <3% of gastric cancers; the majority of genetic changes associated with gastric cancer are acquired. Over the past few decades, advances in technology and high-throughput analysis have improved understanding of the molecular aspects of the pathogenesis of gastric cancer. These aspects are multifaceted and heterogeneous and represent a wide spectrum of several key genetic influences, such as chromosomal instability, microsatellite instability, changes in microRNA profile, somatic gene mutations or functional single nucleotide polymorphisms. These genetic aspects of the pathogenesis of gastric cancer will be addressed in this Review.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ferlay, J. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. International Agency for Research on Cancer [online], (2013).
Arnold, M. et al. Recent trends in incidence of five common cancers in 26 European countries since 1988: Analysis of the European Cancer Observatory. Eur J Cancer. http://dx.doi.org/10.1016/j.ejca.2013.09.002.
Shen, L. et al. Management of gastric cancer in Asia: resource-stratified Guidelines. Lancet Oncol. 14, e535–e547 (2013).
Lauren P. The two histological main types of gastric carcinoma: diffuse and so called intestinal-type carcinoma. Acta Pathol Microbiol Scand. 64, 31–49 (1965).
Correa P. Helicobacter pylori and gastric carcinogenesis. Am. J. Surg. Pathol. 19 (Suppl. 1), S37–S43 (1995).
Yakirevich, E. & Resnick, M. B. Pathology of gastric cancer and its precursor lesions. Gastroenterol. Clin. North Am. 42, 261–284 (2013).
Piazuelo, M. B. & Correa, P. Gastric cancer: Overview. Colomb. Med. (Cali.) 44, 192–201 (2013).
Zanghieri, G. et al. Familial occurrence of gastric cancer in the 2-year experience of a population-based registry. Cancer 66, 2047–2051 (1990).
Sereno, M. et al. Gastric tumours in hereditary cancer syndromes: clinical features, molecular biology and strategies for prevention. Clin. Transl. Oncol. 13, 599–610 (2011).
Fitzgerald, R. C. et al. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J. Med. Genet. 47, 436–444 (2010).
Carneiro, F. Hereditary gastric cancer. Pathologe 33 (Suppl. 2), 231–234 (2012).
Oliveira, C., Pinheiro, H., Figueiredo, J., Seruca, R. & Carneiro, F. E-cadherin-alterations in hereditary disorders with emphasis on dereditary diffuse gastric cancer. Prog. Mol. Biol. Transl. Sci. 116, 337–359 (2013).
Paredes, J. et al. Epithelial E- and P-cadherins: role and clinical significance in cancer. Biochim. Biophys. Acta 1826, 297–311 (2012).
Van Roy, F. Beyond E-cadherin: roles of other cadherin superfamily members in cancer. Nat. Rev. Cancer 14, 121–134 (2014).
Grady, W. M. et al. Methylation of the CDH1 promoter as the second genetic hit in hereditary diffuse gastric cancer. Nat. Genet. 26, 16–17 (2000).
Oliveira, C. et al. Intragenic deletion of CDH1 as the inactivating mechanism of the wild-type allele in an HDGC tumour. Oncogene 23, 2236–2240 (2004).
Oliveira, C. et al. Quantification of epigenetic and genetic 2nd hits in CDH1 during hereditary diffuse gastric cancer syndrome progression. Gastroenterology 136, 2137–2148 (2009).
Mimata, A., Fukamachi, H., Eishi, Y. & Yuasa, Y. Loss of E-cadherin in mouse gastric epithelial cells induces signet ring-like cells, a possible precursor lesion of diffuse gastric cancer. Cancer Sci. 102, 942–950 (2011).
Majewski, I. J. et al. An α-E-catenin (CTNNA1) mutation in hereditary diffuse gastric cancer. J. Pathol. 229, 621–629 (2013).
Schuetz, J. M. et al. Catenin family genes are not commonly mutated in hereditary diffuse gastric cancer. Cancer Epidemiol. Biomarkers Prev. 21, 2272–2274 (2012).
Worthley, D. L. et al. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): a new autosomal dominant syndrome. Gut 61, 774–779 (2012).
Caldas, C. et al. Familial gastric cancer: overview and guidelines for management. J. Med. Genet. 36, 873–880 (1999).
Watson, P. et al. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int. J. Cancer. 123, 444–449 (2008).
Masciari, S. et al. Gastric cancer in individuals with Li–Fraumeni syndrome. Genet. Med. 13, 651–657 (2011).
Hearle, N. et al. Frequency and spectrum of cancers in the Peutz–Jeghers syndrome. Clin. Cancer Res. 12, 3209–3215 (2006).
van Lier, M. G. et al. High cancer risk in Peutz–Jeghers syndrome: a systematic review and surveillance recommendations. Am. J. Gastroenterol. 105, 1258–1264 (2010).
Hirota, W. K. et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest. Endosc. 63, 570–580 (2006).
Cairns S. R. et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 59, 666–689 (2010).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Renovanz, M. & Kim, E. L. Intratumoral heterogeneity, its contribution to therapy resistance and methodological caveats to assessment. Front. Oncol. 4, 142 (2014).
Lengauer, C., Kinzler, K. W. & Vogelstein, B. Genetic instabilities in human cancers. Nature 396, 643–649 (1998).
Hudler, P. Genetic aspects of gastric cancer instability. ScientificWorldJournal http://dx.doi.org/10.1100/2012/761909.
Knuutila, S. et al. DNA copy number amplifications in human neoplasms: review of comparative genomic hybridization studies. Am. J. Pathol. 152, 1107–1123 (1998).
Wu, M. S. et al. Correlation of histologic subtypes and replication error phenotype with comparative genomic hybridization in gastric cancer. Genes Chromosomes Cancer 30, 80–86 (2001).
Kim, K. M. et al. Genetic classification of intestinal-type and diffuse-type gastric cancers based on chromosomal loss and microsatellite instability. Virchows Arch. 443, 491–500 (2003).
Suzuki, K. et al. The genomic damage estimated by arbitrarily primed PCR DNA fingerprinting is useful for the prognosis of gastric cancer. Gastroenterology 125, 1330–1340 (2003).
Weiss, M. M. et al. Genomic alterations in primary gastric adenocarcinomas correlate with clinicopathological characteristics and survival. Cell. Oncol. 26, 307–317 (2004).
Tsukamoto, Y. et al. Genome-wide analysis of DNA copy number alterations and gene expression in gastric cancer. J. Pathol. 216, 471–482 (2008).
Tomioka, N. et al. Array comparative genomic hybridization analysis revealed four genomic prognostic biomarkers for primary gastric cancers. Cancer Genet. Cytogenet. 201, 6–14 (2010).
Grabsch, H. I. & Tan, P. Gastric cancer pathology and underlying molecular mechanisms. Dig. Surg. 30, 150–158 (2013).
Buffart, T. E. et al. Gastric cancers of Western European and African patients show different patterns of genomic instability. BMC Med. Genomics 4, 7 (2011).
Tao, J. et al. CD44–SLC1A2 gene fusions in gastric cancer. Sci. Transl. Med. 3, 77ra30 (2011).
Lee, J. et al. Identification of ROS1 rearrangement in gastric adenocarcinoma. Cancer 119, 1627–1635 (2013).
Davies, K. D. & Doebele, R. C. Molecular pathways: ROS1 fusion proteins in cancer. Clin Cancer Res. 19, 4040–4045 (2013).
Gazvoda, B. et al. Genetic changes in Slovenian patients with gastric adenocarcinoma evaluated in terms of microsatellite DNA. Eur. J. Gastroenterol. Hepatol. 19, 1082–1089 (2007).
Kaur, A. & Dasanu, C. A. Targeting the HER2 pathway for the therapy of lower esophageal and gastric adenocarcinoma. Expert. Opin. Pharmacother. 12, 2493–2503 (2011).
Pazo Cid, R. A. & Antón, A. Advanced HER2-positive gastric cancer: current and future targeted therapies. Crit. Rev. Oncol. Hematol. 85, 350–362 (2013).
Okines A. F. & Cunningham D. Trastuzumab: a novel standard option for patients with HER-2-positive advanced gastric or gastro-oesophageal junction cancer. Therap. Adv. Gastroenterol. 5, 301–318 (2012).
Gunturu, K. S. et al. Gastric cancer and trastuzumab: first biologic therapy in gastric cancer. Ther. Adv. Med. Oncol. 5, 143–151 (2013).
Bang Y. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010).
Yamamoto, H., Imai, K. & Perucho, M. Gastrointestinal cancer of the microsatellite mutator phenotype pathway. J. Gastroenterol. 37, 153–163 (2002).
Ottini, L. et al. Patterns of genomic instability in gastric cancer: clinical implications and perspectives. Ann. Oncol. 17 (Suppl. 7), vii97–vii102 (2006).
Falchetti, M. et al. Gastric cancer with high-level microsatellite instability: target gene mutations, clinicopathologic features, and long-term survival. Hum. Pathol. 39, 925–932 (2008).
Chung, Y. J. et al. Microsatellite instability-associated mutations associate preferentially with the intestinal type of primary gastric carcinomas in a high-risk population. Cancer Res. 56, 4662–4665 (1996).
Yamamoto, H. et al. Gastric cancers of the microsatellite mutator phenotype display characteristic genetic and clinical features. Gastroenterology. 116, 1348–1357 (1999).
Wu, M. S. et al. Distinct clinicopathologic and genetic profiles in sporadic gastric cancer with different mutator phenotypes. Genes Chromosomes Cancer 27, 403–411 (2000).
Fang, W. L. et al. Microsatellite instability is associated with a better prognosis for gastric cancer patients after curative surgery. World J. Surg. 36, 2131–2138 (2012).
Choi, Y. Y. et al. Is microsatellite instability a prognostic marker in gastric cancer? A systematic review with meta-analysis. J. Surg. Oncol. 110, 129–135 (2014).
McColl, K. E. Clinical practice. Helicobacter pylori infection. N. Engl. J. Med. 362, 1597–1604 (2010).
de Martel, C. et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 13, 607–615 (2012).
Calvet, X., Ramírez Lázaro, M. J., Lehours, P. & Mégraud, F. Diagnosis and epidemiology of Helicobacter pylori infection. Helicobacter 18 (Suppl. 1), 5–11 (2013).
Malfertheiner, P., Link, A. & Selgrad, M. Helicobacter pylori: perspectives and time trends. Nat. Rev. Gastroenterol. Hepatol. http://dx.doi.org/10.1038/nrgastro.2014.99.
El-Omar, E. M. et al. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology 109, 681–691 (1995).
El-Omar, E. M. et al. Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology 113, 15–24 (1997).
El-Omar, E. M. The importance of interleukin 1β in Helicobacter pylori associated disease. Gut 48, 743–747 (2001).
Tu, S. et al. Overexpression of interleukin-1β induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell 14, 408–419 (2008).
El-Omar, E. M. et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404, 398–402 (2000).
Kimang'a, A. N. IL-1B-511 allele T and IL-1RN-L/L play a pathological role in Helicobacter pylori (H. pylori) disease outcome in the African population. Ethiop. J. Health Sci. 22, 163–169 (2012).
Zhao, J. D. et al. Associations between interleukin-1 polymorphisms and gastric cancers among three ethnicities. World J. Gastroenterol. 18, 7093–7099 (2012).
Kato, S. et al. Association of the interleukin-1β genetic polymorphism and gastric cancer risk in Japanese. J. Gastroenterol. 36, 696–699 (2001).
Kamangar, F., Cheng, C., Abnet, C. C. & Rabkin, C. S. Interleukin-1B polymorphisms and gastric cancer risk—a meta-analysis. Cancer Epidemiol. Biomarkers Prev. 15, 1920–1928 (2006).
Garcia-Gonzalez, M. A. et al. Gastric cancer susceptibility is not linked to pro-and anti-inflammatory cytokine gene polymorphisms in whites: a Nationwide Multicenter Study in Spain. Am. J. Gastroenterol. 102, 1878–1892 (2007).
Sitarz, R. et al. IL-1B-31T>C promoter polymorphism is associated with gastric stump cancer but not with early onset or conventional gastric cancers. Virchows Arch. 453, 249–255 (2008).
Murphy, G. et al. Association of gastric disease with polymorphisms in the inflammatory-related genes IL-1B, IL-1RN, IL-10, TNF and TLR4. Eur. J. Gastroenterol. Hepatol. 21, 630–635 (2009).
Persson, C. et al. Interleukin 1-β gene polymorphisms and risk of gastric cancer in Sweden. Scand. J. Gastroenterol. 44, 339–345 (2009).
Wex, T. et al. Interleukin 1 β (IL1B) gene polymorphisms are not associated with gastric carcinogenesis in Germany. Anticancer Res. 30, 505–511 (2010).
He, B. et al. Interleukin 1 β (IL1B) promoter polymorphism and cancer risk: evidence from 47 published studies. Mutagenesis 26, 637–642 (2011).
Camargo, M. C. et al. Interleukin-1β and interleukin-1 receptor antagonist gene polymorphisms and gastric cancer: a meta-analysis. Cancer Epidemiol. Biomarkers Prev. 15, 1674–1687 (2006).
Vincenzi, B. et al. Interleukin 1β-511T gene (IL1β) polymorphism is correlated with gastric cancer in the Caucasian population: results from a meta-analysis. Oncol. Rep. 20, 1213–1220 (2008).
Loh, M. et al. Meta-analysis of genetic polymorphisms and gastric cancer risk: variability in associations according to race. Eur. J. Cancer 45, 2562–2568 (2009).
Xue, H., Lin, B., Ni, P., Xu, H. & Huang, G. Interleukin-1B and interleukin-1 RN polymorphisms and gastric carcinoma risk: a meta-analysis. J. Gastroenterol. Hepatol. 25, 1604–1617 (2010).
Persson, C., Canedo, P., Machado, J. C., El-Omar, E. M. & Forman, D. Polymorphisms in inflammatory response genes and their association with gastric cancer: A HuGE systematic review and meta-analyses. Am. J. Epidemiol. 173, 259–270 (2011).
Zou, W. & Restifo, N. P. TH17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol. 10, 248–256 (2010).
Bettelli, E., Oukka, M. & Kuchroo, V. K. TH-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 8, 345–350 (2007).
Maruyama, T. et al. Distribution of TH17 cells and FoxP3(+) regulatory T cells in tumor-infiltrating lymphocytes, tumor-draining lymph nodes and peripheral blood lymphocytes in patients with gastric cancer. Cancer Sci. 101, 1947–1954 (2010).
Iida, T. et al. Tumor-infiltrating CD4+ Th17 cells produce IL-17 in tumor microenvironment and promote tumor progression in human gastric cancer. Oncol. Rep. 25, 1271–1277 (2011).
Meng, X. Y., Zhou, C. H., Ma, J., Jiang, C. & Ji, P. Expression of interleukin-17 and its clinical significance in gastric cancer patients. Med. Oncol. 29, 3024–3028 (2012).
Zhuang, Y. et al. CD8+ T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology 143, 951–962 (2012).
Shibata, T., Tahara, T., Hirata, I. & Arisawa, T. Genetic polymorphism of interleukin-17A and -17 F genes in gastric carcinogenesis. Hum. Immunol. 70, 547–551 (2009).
Qinghai, Z., Yanying, W., Yunfang, C., Xukui, Z. & Xiaoqiao, Z. Effect of interleukin-17A and interleukin-17F gene polymorphisms on the risk of gastric cancer in a Chinese population. Gene 537, 328–332 (2014).
Rafiei, A. et al. Polymorphism in the interleukin-17A promoter contributes to gastric cancer. World J. Gastroenterol. 19, 5693–5699 (2013).
Zhang, X., Zheng, L., Sun, Y. & Zhang, X. Analysis of the association of interleukin-17 gene polymorphisms with gastric cancer risk and interaction with Helicobacter pylori infection in a Chinese population. Tumour Biol. 35, 1575–1580 (2014).
Kabir, S. The role of interleukin-17 in the Helicobacter pylori induced infection and immunity. Helicobacter 16, 1–8 (2011).
Zhou, R. M. et al. Polymorphisms in promoter region of FAS and FASL gene and risk of cardia gastric adenocarcinoma. J. Gastroenterol. Hepatol. 25, 555–561 (2010).
Cui, Y., Xue, H., Lin, B., Ni, P. & Fang, J. Y. A meta-analysis of CDH1 C-160A genetic polymorphism and gastric cancer risk. DNA Cell Biol. 30, 937–945 (2011).
Hyland, P. L. et al. Genetic variants in fas signaling pathway genes and risk of gastric cancer. Int. J. Cancer. 134, 822–831 (2014).
Xu, Q., Chen, M. Y., He, C. Y., Sun, L. P. & Yuan, Y. Promoter polymorphisms in trefoil factor 2 and trefoil factor 3 genes and susceptibility to gastric cancer and atrophic gastritis among Chinese population. Gene 529, 104–112 (2013).
Ng, M. T. et al. Increase in NF-κB binding affinity of the variant C allele of the toll-like receptor 9 −1237T/C polymorphism is associated with Helicobacter pylori-induced gastric disease. Infect. Immun. 78, 1345–1352 (2010).
Huang, L. et al. Polymorphisms of the TLR4 gene and risk of gastric cancer. Gene 537, 46–50 (2014).
Companioni, O. et al. Polymorphisms of Helicobacter pylori signaling pathway genes and gastric cancer risk in the European Prospective Investigation into Cancer-Eurgast cohort. Int. J. Cancer. 134, 92–101 (2014).
Sakamoto, H. et al. Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer. Nat. Genet. 40, 730–740 (2008).
Matsuo, K. et al. Association of prostate stem cell antigen gene polymorphisms with the risk of stomach cancer in Japanese. Int. J. Cancer. 125, 1961–1964 (2009).
Wu, C. et al. Two genetic variants in prostate stem cell antigen and gastric cancer susceptibility in a Chinese population. Mol. Carcinog. 48, 1131–1138 (2009).
Lu, Y. et al. Genetic variation of PSCA gene is associated with the risk of both diffuse- and intestinal-type gastric cancer in a Chinese population. Int. J. Cancer. 127, 2183–2189 (2010).
Zeng, Z. et al. Polymorphisms in prostate stem cell antigen gene rs2294008 increase gastric cancer risk in Chinese. Mol. Carcinog. 50, 353–358 (2011).
Lochhead, P. et al. Genetic variation in the prostate stem cell antigen gene and upper gastrointestinal cancer in white individuals. Gastroenterology 140, 435–441 (2011).
Sala, N. et al. Prostate stem-cell antigen gene is associated with diffuse and intestinal gastric cancer in Caucasians: results from the EPIC-EURGAST study. Int. J. Cancer 130, 2417–2427 (2012).
Qiao, L. & Feng, Y. Genetic variations of prostate stem cell antigen (PSCA) contribute to the risk of gastric cancer for Eastern Asians: a meta-analysis based on 16792 individuals. Gene 493, 83–91 (2012).
Shi, D. et al. The PSCA polymorphisms derived from genome-wide association study are associated with risk of gastric cancer: a meta-analysis. J. Cancer Res. Clin. Oncol. 138, 1339–1345 (2012).
Wang, T., Zhang, L., Li, H., Wang, B. & Chen, K. Prostate stem cell antigen polymorphisms and susceptibility to gastric cancer: a systematic review and meta-analysis. Cancer Epidemiol. Biomarkers Prev. 21, 843–850 (2012).
Zhang, T., Chen, Y. N., Wang, Z., Chen, J. Q. & Huang, S. Effect of PSCA gene polymorphisms on gastric cancer risk and survival prediction: a meta-analysis. Exp. Ther. Med. 4, 158–164 (2012).
Gu, X., Zhang, W., Xu, L. & Cai, D. Quantitative assessment of the influence of prostate stem cell antigen polymorphisms on gastric cancer risk. Tumour Biol. 35, 2167–2174 (2014).
Wang, M. et al. Genetic variant in PSCA predicts survival of diffuse-type gastric cancer in a Chinese population. Int. J. Cancer. 129, 1207–1213 (2011).
Kufe, D. W. Mucins in cancer: Function, prognosis and therapy. Nat. Rev. Cancer. 9, 874–885 (2009).
Senapati, S., Das, S. & Batra, S. K. Mucin-interacting proteins: from function to therapeutics. Trends Biochem. Sci. 35, 236–245 (2010).
Boltin, D. & Niv, Y. Mucins in gastric cancer—an update. J. Gastrointest. Dig. Syst. 3, 15519 (2013).
Kufe, D. W. MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. Oncogene 32, 1073–1081 (2013).
Mukhopadhyay, P. et al. Mucins in the pathogenesis of breast cancer: implications in diagnosis, prognosis and therapy. Biochim. Biophys. Acta. 1815, 224–240 (2011).
Roulois, D., Grégoire, M. & Fonteneau, J. F. MUC1-specific cytotoxic T lymphocytes in cancer therapy: induction and challenge. Biomed. Res. Int. 2013, 871936 (2013).
Namikawa, T. & Hanazaki, K. Mucin phenotype of gastric cancer and clinicopathology of gastric-type differentiated adenocarcinoma. World J. Gastroenterol. 16, 4634–4639 (2010).
Hwang, I. et al. Prognostic significance of membrane-associated mucins 1 and 4 in gastric adenocarcinoma. Exp. Ther. Med. 4, 311–316 (2012).
Tamura, Y. et al. MUC4 and MUC1 expression in adenocarcinoma of the stomach correlates with vessel invasion and lymph node metastasis: an immunohistochemical study of early gastric cancer. PLoS ONE 7, e49251 (2012).
Xu, Q. et al. The co-expression of functional gastric proteins in dynamic gastric diseases and its clinical significance. BMC Clin. Pathol. 13, 21 (2013).
Silva, F. et al. MUC1 gene polymorphism in the gastric carcinogenesis pathway. Eur. J. Hum. Genet. 9, 548–552 (2001).
Xu, Q. et al. Risk of gastric cancer is associated with the MUC1 568 A/G polymorphism. Int. J. Oncol. 35, 1313–1320 (2009).
Abnet, C. C. et al. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat. Genet. 42, 764–767 (2010).
Jia, Y. et al. A comprehensive analysis of common genetic variation in MUC1, MUC5AC, MUC6 genes and risk of stomach cancer. Cancer Causes Control 21, 313–321 (2010).
Saeki, N. et al. A functional single nucleotide polymorphism in mucin 1, at chromosome 1q22, determines susceptibility to diffuse-type gastric cancer. Gastroenterology 140, 892–902 (2011).
Zhang, H. et al. Genetic variants at 1q22 and 10q23 reproducibly associated with gastric cancer susceptibility in a Chinese population. Carcinogenesis 32, 848–852 (2011).
Palmer, A. J. et al. Genetic variation in C20orf54, PLCE1 and MUC1 and the risk of upper gastrointestinal cancers in Caucasian populations. Eur. J. Cancer Prev. 21, 541–544 (2012).
Song, H. R. et al. Common genetic variants at 1q22 and 10q23 and gastric cancer susceptibility in a Korean population. Tumour Biol. 35, 3133–3137 (2014).
Zheng, L. et al. Functional polymorphism rs4072037 in MUC1 gene contributes to the susceptibility to gastric cancer: evidence from pooled 6,580 cases and 10,324 controls. Mol. Biol. Rep. 40, 5791–5796 (2013).
Ng, W. et al. Genetic regulation of MUC1 alternative splicing in human tissues. Br. J. Cancer 99, 978–985 (2008).
Guang, W. et al. Muc1 cell surface mucin attenuates epithelial inflammation in response to a common mucosal pathogen. J. Biol. Chem. 285, 20547–20557 (2010).
He, C., Chen, M., Liu, J. & Yuan, Y. Host genetic factors respond to pathogenic step-specific virulence factors of Helicobacter pylori in gastric carcinogenesis. Mutat. Res. 759, 14–26 (2014).
Ueno, K. et al. MUC1 mucin is a negative regulator of Toll-like receptor signaling. Am. J. Respir. Cell. Mol. Biol. 38, 263–268 (2008).
Sheng, Y. H. et al. MUC1 and MUC13 differentially regulate epithelial inflammation in response to inflammatory and infectious stimuli. Mucosal Immunol. 6, 557–568 (2013).
Marín, F. et al. Genetic variation in MUC1, MUC2 and MUC6 genes and evolution of gastric cancer precursor lesions in a long-term follow-up in a high-risk area in Spain. Carcinogenesis 33, 1072–1080 (2012).
Saeki, N., Ono, H., Sakamoto, H. & Yoshida, T. Genetic factors related to gastric cancer susceptibility identified using a genome-wide association study. Cancer Sci. 104, 1–8 (2013).
Shi, Y. et al. A genome-wide association study identifies new susceptibility loci for non-cardiagastric cancer at 3q13.31 and 5p13.1. Nat. Genet. 43, 1215–1218 (2011).
Kang, M. et al. Genetic variation rs10484761 on 6p21.1 derived from a genome-wide association study is associated with gastric cancer survival in a Chinese population. Gene 536, 59–64 (2014).
Liang, H. & Kim, Y. H. Identifying molecular drivers of gastric cancer through next generation sequencing. Cancer Letters 340, 241–246 (2013).
Zang, Z. J. et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat. Genet. 44, 570–574 (2012).
Wang K. et al. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat. Genet. 43, 1219–1223 (2011).
Zang, Z. J. et al. Genetic and structural variation in the gastric cancer kinome revealed through targeted deep sequencing. Cancer Res. 71, 29–39 (2011).
Tan, I. B. et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology 141, 476–485 (2011).
Lei, Z. et al. Identification of molecular subtypes of gastric cancer with different responses to PI3-kinase inhibitors and 5-fluorouracil. Gastroenterology 145, 554–565 (2013).
Deng N. et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 61, 673–684 (2012).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
Kakiuchi, M. et al. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat. Genet. 46, 583–587 (2014).
The National Institutes of Health. The Cancer Genome Atlas [online], (2014).
Wellcome Trust Sanger Institute. Cancer Genome Project [online], (2014).
International Cancer Genome Consortium (ICGC). International Cancer Genome Projects [online], (2014).
The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature http://dx.doi.org/10.1038/nature13480.
Gigek, C. O. et al. Epigenetic mechanisms in gastric cancer. Epigenomics 4, 279–294 (2012).
Zouridis, H. et al. Methylation subtypes and large-scale epigenetic alterations in gastric cancer. Sci. Transl. Med. 4, 156ra140 (2012).
Calcagno, D. Q. et al. DNA and histone methylation in gastric carcinogenesis. World J. Gastroenterol. 19, 1182–1192 (2013).
Qu, Y., Dang, S. & Hou, P. Gene methylation in gastric cancer. Clin. Chim. Acta 424, 53–65 (2013).
Ling, H., Fabbri, M. & Calin, G. A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 12, 847–865 (2013).
Wu, W. K. et al. MicroRNA dysregulation in gastric cancer: a new player enters the game. Oncogene 29, 5761–5771 (2010).
Link, A., Kupcinskas, J., Wex, T. & Malfertheiner, P. Macro-role of microRNA in gastric cancer. Dig. Dis. 30, 255–267 (2012).
Song, J. H. & Meltzer, S. J. MicroRNAs in pathogenesis, diagnosis, and treatment of gastroesophageal cancers. Gastroenterology 143, 35–47 (2012).
Song, S. & Ajani, J. A. The role of microRNAs in cancers of the upper gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 10, 109–118 (2013).
Zhang, X. et al. MicroRNA-181a promotes gastric cancer by negatively regulating tumor suppressor KLF6. Tumour Biol. 33, 1589–1597 (2012).
Bou Kheir, T. et al. miR-449 inhibits cell proliferation and is down-regulated in gastric cancer. Mol. Cancer 10, 29 (2011).
Li, X., Zhang, Y., Ding, J, Wu, K. & Fan, D. Survival prediction of gastric cancer by a seven-microRNA signature. Gut 59, 579–585 (2010).
Ueda, T. et al. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 11, 136–146 (2010).
Wang, F. et al. MicroRNAs as promising biomarkers for gastric cancer. Cancer Biomark. 11, 259–267 (2012).
Xia, L. et al. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int. J. Cancer 123, 372–379 (2008).
Hummel, R., Hussey, D. J. & Haier, J. MicroRNAs: predictors and modifiers of chemo- and radiotherapy in different tumour types. Eur. J. Cancer 46, 298–311 (2010).
Wu, H. et al. Regulation of microtubule-associated protein tau (MAPT) by miR-34c-5p determines the chemosensitivity of gastric cancer to paclitaxel. Cancer Chemother. Pharmacol. 71, 1159–1171 (2013).
Wang, Q. et al. Polymorphisms at the microRNA binding-site of the stem cell marker gene CD133 modify susceptibility to and survival of gastric cancer. Mol. Carcinog. http://dx.doi.org/10.1002/mc.22113.
Xu, Y. et al. The miR-184 binding-site rs8126 T>C polymorphism in TNFAIP2 is associated with risk of gastric cancer. PLoS ONE 8, e64973 (2013).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
McLean, M., El-Omar, E. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol 11, 664–674 (2014). https://doi.org/10.1038/nrgastro.2014.143
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2014.143
This article is cited by
-
Development and validation of a nomogram to predict cancer-specific survival of elderly patients with unresected gastric cancer who received chemotherapy
Scientific Reports (2024)
-
Development and validation of nomograms for predicting overall survival and cancer-specific survival in elderly patients with locally advanced gastric cancer: a population-based study
BMC Gastroenterology (2023)
-
PWSC: a novel clustering method based on polynomial weight-adjusted sparse clustering for sparse biomedical data and its application in cancer subtyping
BMC Bioinformatics (2023)
-
MESP2 binds competitively to TCF4 to suppress gastric cancer progression by regulating the SKP2/p27 axis
Cell Death Discovery (2023)
-
A multi-institutional study to evaluate the feasibility of next-generation sequencing and genomic analysis using formalin-fixed, paraffin-embedded biopsies of gastric cancer
Gastric Cancer (2023)