Key Points
-
The availability of tools that can accurately measure response is critical for the selection of patients with rectal cancer who can be offered organ-saving treatment
-
After preoperative chemoradiotherapy, endorectal ultrasonography and MRI are limited in the detection of small tumour remnants within the fibrotic tumour bed
-
Functional MRI combines detailed information on tumour morphology with that on the tumour biological microenvironment and provides comprehensive information on tumour heterogeneity
-
Diffusion-weighted imaging, as a qualitative measure of response is promising for the assessment of clinical complete response, but the evidence so far is mainly from single-centre expert studies
-
It is too early to conclude that perfusion magnetic resonance biomarkers could have a role as a measure of response, and many issues need to be resolved
-
The future of response evaluation with imaging lies in further exploitation of all information that is within medical images
Abstract
Guidelines recommend MRI as part of the staging work-up of patients with rectal cancer because it can identify high-risk groups requiring preoperative treatment. Phenomenal tumour responses have been observed with current chemoradiotherapy regimens—even complete regression in 25% of patients. For these patients, the options of organ-saving treatment as an alternative to surgery are now discussed, and critical for this approach is the availability of tools that can accurately measure response. The value of MRI in rectal cancer staging is established, but the role of MRI for the selection of patients for organ-saving treatment is debatable, because MRI is not able to accurately assess tumour response to preoperative chemoradiotherapy (owing to its reliance on morphological changes). Functional MRI is emerging in the field of oncology. It combines information on detailed anatomy with that of tumour biology, providing comprehensive information on tumour heterogeneity and its changes as a result of treatment. This Review provides knowledge on the strengths and weaknesses of MRI for response assessment after chemoradiotherapy in rectal cancer and on its ability to predict tumour response at the time of primary diagnosis. It elaborates on new functional magnetic resonance technology and discusses whether this and new postprocessing approaches have the potential to improve prediction and assessment of response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
van de Velde, C. J. et al. EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum. Eur. J. Cancer 50, 1.e1–1.e34 (2014).
Engelen, S. M. et al. Modern multidisciplinary treatment of rectal cancer based on staging with magnetic resonance imaging leads to excellent local control, but distant control remains a challenge. Eur. J. Cancer 49, 2311–2320 (2013).
Maas, M. et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 11, 835–844 (2010).
Maas, M. et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J. Clin. Oncol. 29, 4633–4640 (2011).
Habr-Gama, A., Perez, R. O., Sao Juliao, G. P., Proscurshim, I. & Gama-Rodrigues, J. Nonoperative approaches to rectal cancer: a critical evaluation. Semin. Radiat. Oncol. 21, 234–239 (2011).
Habr-Gama, A. et al. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis. Colon Rectum 53, 1692–1698 (2010).
Perez, R. O. et al. Role of biopsies in patients with residual rectal cancer following neoadjuvant chemoradiation after downsizing: can they rule out persisting cancer? Colorectal Dis. 14, 714–720 (2012).
Lambin, P. et al. Predicting outcomes in radiation oncology—multifactorial decision support systems. Nat. Rev. Clin. Oncol. 10, 27–40 (2013).
van Stiphout, R. G. et al. Development and external validation of a predictive model for pathological complete response of rectal cancer patients including sequential PET-CT imaging. Radiother. Oncol. 98, 126–133 (2011).
MacGregor, T. P., Maughan, T. S. & Sharma, R. A. Pathological grading of regression following neoadjuvant chemoradiation therapy: the clinical need is now. J. Clin. Pathol. 65, 867–871 (2012).
Sloothaak, D. A. et al. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br. J. Surg. 100, 933–939 (2013).
Vliegen, R. F. et al. Mesorectal fascia invasion after neoadjuvant chemotherapy and radiation therapy for locally advanced rectal cancer: accuracy of MR imaging for prediction. Radiology 246, 454–462 (2008).
Kulkarni, T. et al. Magnetic resonance imaging in rectal cancer downstaged using neoadjuvant chemoradiation: accuracy of prediction of tumour stage and circumferential resection margin status. Colorectal Dis. 10, 479–489 (2008).
MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ 333, 779 (2006).
Beets-Tan, R. G. & Beets, G. L. Local staging of rectal cancer: review of imaging. J. Magn. Reson. Imaging 33, 1012–1019 (2011).
Vanagunas, A., Lin, D. E. & Stryker, S. J. Accuracy of endoscopic ultrasound for restaging rectal cancer following neoadjuvant chemoradiation therapy. Am. J. Gastroenterol. 99, 109–112 (2004).
Napoleon, B. et al. Accuracy of endosonography in the staging of rectal cancer treated by radiotherapy. Br. J. Surg. 78, 785–788 (1991).
Radovanovic, Z., Breberina, M., Petrovic, T., Golubovic, A. & Radovanovic, D. Accuracy of endorectal ultrasonography in staging locally advanced rectal cancer after preoperative chemoradiation. Surg. Endosc. 22, 2412–2415 (2008).
Huh, J. W., Park, Y. A., Jung, E. J., Lee, K. Y. & Sohn, S. K. Accuracy of endorectal ultrasonography and computed tomography for restaging rectal cancer after preoperative chemoradiation. J. Am. Coll. Surg. 207, 7–12 (2008).
Pastor, C. et al. Accuracy of endoscopic ultrasound to assess tumor response after neoadjuvant treatment in rectal cancer: can we trust the findings? Dis. Colon Rectum 54, 1141–1146 (2011).
van der Paardt, M. P., Zagers, M. B., Beets-Tan, R. G., Stoker, J. & Bipat, S. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology 269, 101–112 (2013).
Dresen, R. C. et al. Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part I. Are we able to predict tumor confined to the rectal wall? Radiology 252, 71–80 (2009).
Barbaro, B. et al. Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology 250, 730–739 (2009).
Curvo-Semedo, L. et al. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy—conventional MR volumetry versus diffusion-weighted MR imaging. Radiology 260, 734–743 (2011).
Ha, H. I., Kim, A. Y., Yu, C. S., Park, S. H. & Ha, H. K. Locally advanced rectal cancer: diffusion-weighted MR tumour volumetry and the apparent diffusion coefficient for evaluating complete remission after preoperative chemoradiation therapy. Eur. Radiol. 23, 3345–3353 (2013).
Padhani, A. R. & Miles, K. A. Multiparametric imaging of tumor response to therapy. Radiology 256, 348–364 (2010).
Figueiras, R. G. et al. The role of functional imaging in colorectal cancer. AJR Am. J. Roentgenol. 195, 54–66 (2010).
Li, S. P. & Padhani, A. R. Tumor response assessments with diffusion and perfusion MRI. J. Magn. Reson. Imaging 35, 745–763 (2012).
Kuhl, C. K. et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology 211, 101–110 (1999).
Leach, M. O. et al. The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: issues and recommendations. Br. J. Cancer 92, 1599–1610 (2005).
Tofts, P. S. et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J. Magn. Reson. Imaging 10, 223–232 (1999).
Oberholzer, K. et al. Rectal cancer: assessment of response to neoadjuvant chemoradiation by dynamic contrast-enhanced MRI. J. Magn. Reson. Imaging 38, 119–126 (2013).
Gollub, M. J. et al. Dynamic contrast enhanced-MRI for the detection of pathological complete response to neoadjuvant chemotherapy for locally advanced rectal cancer. Eur. Radiol. 22, 821–831 (2012).
Lim, J. S. et al. Perfusion MRI for the prediction of treatment response after preoperative chemoradiotherapy in locally advanced rectal cancer. Eur. Radiol. 22, 1693–1700 (2012).
Lambregts, D. M. et al. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann. Surg. Oncol. 18, 2224–2231 (2011).
Sun, Y. S. et al. Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion-weighted MR imaging for early detection of tumor histopathologic downstaging. Radiology 254, 170–178 (2010).
Ippolito, D. et al. Response to neoadjuvant therapy in locally advanced rectal cancer: assessment with diffusion-weighted MR imaging and 18FDG PET/CT. Abdom. Imaging 37, 1032–1040 (2012).
Jung, S. H. et al. Predicting response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer: diffusion-weighted 3 Tesla MR imaging. J. Magn. Reson. Imaging 35, 110–116 (2012).
Lambrecht, M. et al. Value of diffusion-weighted magnetic resonance imaging for prediction and early assessment of response to neoadjuvant radiochemotherapy in rectal cancer: preliminary results. Int. J. Radiat. Oncol. Biol. Phys. 82, 863–870 (2012).
Monguzzi, L. et al. Locally advanced rectal cancer: value of ADC mapping in prediction of tumor response to radiochemotherapy. Eur. J. Radiol. 82, 234–240 (2013).
Intven, M., Reerink, O. & Philippens, M. E. Diffusion-weighted MRI in locally advanced rectal cancer: pathological response prediction after neo-adjuvant radiochemotherapy. Strahlenther. Onkol. 189, 117–122 (2013).
Kim, S. H. et al. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology 253, 116–125 (2009).
Kim, S. H., Lee, J. Y., Lee, J. M., Han, J. K. & Choi, B. I. Apparent diffusion coefficient for evaluating tumour response to neoadjuvant chemoradiation therapy for locally advanced rectal cancer. Eur. Radiol. 21, 987–995 (2011).
Song, I. et al. Value of diffusion-weighted imaging in the detection of viable tumour after neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer: comparison with T2 weighted and PET/CT imaging. Br. J. Radiol. 85, 577–586 (2012).
Yu, S. K., Tait, D., Chau, I. & Brown, G. MRI predictive factors for tumor response in rectal cancer following neoadjuvant chemoradiation therapy—implications for induction chemotherapy? Int. J. Radiat. Oncol. Biol. Phys. 87, 505–511 (2013).
Chang, G. J. et al. Pretreatment high-resolution rectal MRI and treatment response to neoadjuvant chemoradiation. Dis. Colon Rectum 55, 371–377 (2012).
Oberholzer, K. et al. Rectal cancer: mucinous carcinoma on magnetic resonance imaging indicates poor response to neoadjuvant chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 82, 842–848 (2012).
Hermans, R. et al. Tumor perfusion rate determined noninvasively by dynamic computed tomography predicts outcome in head-and-neck cancer after radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 57, 1351–1356 (2003).
Zahra, M. A., Hollingsworth, K. G., Sala, E., Lomas, D. J. & Tan, L. T. Dynamic contrast-enhanced MRI as a predictor of tumour response to radiotherapy. Lancet Oncol. 8, 63–74 (2007).
George, M. L. et al. Non-invasive methods of assessing angiogenesis and their value in predicting response to treatment in colorectal cancer. Br. J. Surg. 88, 1628–1636 (2001).
Devries, A. F. et al. Tumor microcirculation evaluated by dynamic magnetic resonance imaging predicts therapy outcome for primary rectal carcinoma. Cancer Res. 61, 2513–2516 (2001).
de Vries, A. et al. Monitoring of tumor microcirculation during fractionated radiation therapy in patients with rectal carcinoma: preliminary results and implications for therapy. Radiology 217, 385–391 (2000).
Kremser, C., Trieb, T., Rudisch, A., Judmaier, W. & de Vries, A. Dynamic T(1) mapping predicts outcome of chemoradiation therapy in primary rectal carcinoma: sequence implementation and data analysis. J. Magn. Reson. Imaging 26, 662–671 (2007).
DeVries, A. F. et al. Tumor microcirculation and diffusion predict therapy outcome for primary rectal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 56, 958–965 (2003).
Kim, Y. C. et al. Comparison of diffusion-weighted MRI and MR volumetry in the evaluation of early treatment outcomes after preoperative chemoradiotherapy for locally advanced rectal cancer. J. Magn. Reson. Imaging 34, 570–576 (2011).
Barbaro, B. et al. Diffusion-weighted magnetic resonance imaging in monitoring rectal cancer response to neoadjuvant chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 83, 594–599 (2012).
Lambrecht, M. et al. The use of FDG-PET/CT and diffusion-weighted magnetic resonance imaging for response prediction before, during and after preoperative chemoradiotherapy for rectal cancer. Acta Oncol. 49, 956–963 (2010).
Beets-Tan, R. G. et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur. Radiol. 23, 2522–2531 (2013).
Kim, M. J. et al. Detection of rectal cancer and response to concurrent chemoradiotherapy by proton magnetic resonance spectroscopy. Magn. Reson. Imaging 30, 848–853 (2012).
Kyriazi, S. et al. Metastatic ovarian and primary peritoneal cancer: assessing chemotherapy response with diffusion-weighted MR imaging—value of histogram analysis of apparent diffusion coefficients. Radiology 261, 182–192 (2011).
Wilmes, L. J. et al. High-resolution diffusion-weighted imaging for monitoring breast cancer treatment response. Acad. Radiol. 20, 581–589 (2013).
Kluza, E. et al. T2 weighted signal intensity evolution may predict pathological complete response after treatment for rectal cancer. Eur. Radiol. 23, 253–261 (2013).
Carbone, S. F. et al. Assessment of response to chemoradiation therapy in rectal cancer using MR volumetry based on diffusion-weighted data sets: a preliminary report. Radiol. Med. 117, 1112–1124 (2012).
Castellano, G., Bonilha, L., Li, L. M. & Cendes, F. Texture analysis of medical images. Clin. Radiol. 59, 1061–1069 (2004).
Goh, V. et al. Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology 261, 165–171 (2011).
Ng, F., Ganeshan, B., Kozarski, R., Miles, K. A. & Goh, V. Assessment of primary colorectal cancer heterogeneity by using whole-tumor texture analysis: contrast-enhanced CT texture as a biomarker of 5-year survival. Radiology 266, 177–184 (2013).
Miles, K. A., Ganeshan, B., Griffiths, M. R., Young, R. C. & Chatwin, C. R. Colorectal cancer: texture analysis of portal phase hepatic CT images as a potential marker of survival. Radiology 250, 444–452 (2009).
Freeborough, P. A. & Fox, N. C. MR image texture analysis applied to the diagnosis and tracking of Alzheimer's disease. IEEE Trans. Med. Imaging 17, 475–479 (1998).
Lerski, R. A. et al. MR image texture analysis—an approach to tissue characterization. Magn. Reson. Imaging 11, 873–887 (1993).
Sinha, S. et al. Multifeature analysis of Gd-enhanced MR images of breast lesions. J. Magn. Reson. Imaging 7, 1016–1026 (1997).
Chen, W., Giger, M. L., Li, H., Bick, U. & Newstead, G. M. Volumetric texture analysis of breast lesions on contrast-enhanced magnetic resonance images. Magn. Reson. Med. 58, 562–571 (2007).
Kjaer, L., Ring, P., Thomsen, C. & Henriksen, O. Texture analysis in quantitative MR imaging. Tissue characterisation of normal brain and intracranial tumours at 1.5 T. Acta Radiol. 36, 127–135 (1995).
Gibbs, P. & Turnbull, L. W. Textural analysis of contrast-enhanced MR images of the breast. Magn. Reson. Med. 50, 92–98 (2003).
Ahmed, A., Gibbs, P., Pickles, M. & Turnbull, L. Texture analysis in assessment and prediction of chemotherapy response in breast cancer. J. Magn. Reson. Imaging 38, 89–101 (2013).
Ganeshan, B. et al. Non-small cell lung cancer: histopathologic correlates for texture parameters at CT. Radiology 266, 326–336 (2013).
Author information
Authors and Affiliations
Contributions
R.G.H.B.-T. contributed to researching data, discussion of content, writing and reviewing/editing the manuscript. G.L.B. contributed to discussion of content, writing and reviewing/editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Beets-Tan, R., Beets, G. MRI for assessing and predicting response to neoadjuvant treatment in rectal cancer. Nat Rev Gastroenterol Hepatol 11, 480–488 (2014). https://doi.org/10.1038/nrgastro.2014.41
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2014.41
This article is cited by
-
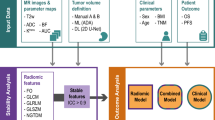
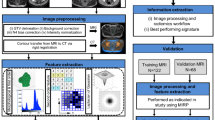
Multiphase and multiparameter MRI-based radiomics for prediction of tumor response to neoadjuvant therapy in locally advanced rectal cancer
Radiation Oncology (2023)
-
Fundamentals of Bowel Cancer for Biomedical Engineers
Annals of Biomedical Engineering (2023)
-
A nomogram model based on MRI and radiomic features developed and validated for the evaluation of lymph node metastasis in patients with rectal cancer
Abdominal Radiology (2022)
-
The efficiency of 18F-FDG-PET/CT in the assessment of tumor response to preoperative chemoradiation therapy for locally recurrent rectal cancer
BMC Cancer (2021)
-
MRI-based clinical-radiomics model predicts tumor response before treatment in locally advanced rectal cancer
Scientific Reports (2021)