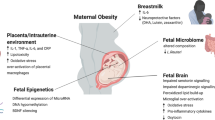
Key Points
-
Exposure to maternal overnutrition or undernutrition increases susceptibility to NAFLD in childhood and hastens progression to NASH across the lifespan, especially when offspring are exposed postnatally to a high-fat (Western-style) diet
-
Maternal obesity and insulin resistance acting through the placenta produce intrauterine exposures including increased fetal insulin, lipids, inflammation and possibly hypoxia, which cause developmental programming of hepatosteatosis before birth
-
Programming of hepatic pathways including de novo lipogenesis, oxidative stress, mitochondrial dysfunction and inflammation persists in juvenile offspring born to and breastfed by mothers with obesity, despite weaning to a healthy diet
-
Pioneering microorganisms transmitted to the neonate at birth and microorganisms and bioactive milk products transferred during breastfeeding educate the infant immune system with lifelong implications for disease susceptibility
-
Pro-inflammatory and pro-remodelling phenotypes in liver macrophages might be programmed in infancy through alteration of intracellular metabolic and epigenetic pathways, increasing responsiveness to subsequent inflammatory stimuli
-
Supplements in preclinical models targeting novel pathways and receptors might be emerging therapies for use in tandem with a dietary shift to more complex carbohydrates during pregnancy
Abstract
Changes in the maternal environment leading to an altered intrauterine milieu can result in subtle insults to the fetus, promoting increased lifetime disease risk and/or disease acceleration in childhood and later in life. Particularly worrisome is that the prevalence of NAFLD is rapidly increasing among children and adults, and is being diagnosed at increasingly younger ages, pointing towards an early-life origin. A wealth of evidence, in humans and non-human primates, suggests that maternal nutrition affects the placenta and fetal tissues, leading to persistent changes in hepatic metabolism, mitochondrial function, the intestinal microbiota, liver macrophage activation and susceptibility to NASH postnatally. Deleterious exposures in utero include fetal hypoxia, increased nutrient supply, inflammation and altered gut microbiota that might produce metabolic clues, including fatty acids, metabolites, endotoxins, bile acids and cytokines, which prime the infant liver for NAFLD in a persistent manner and increase susceptibility to NASH. Mechanistic links to early disease pathways might involve shifts in lipid metabolism, mitochondrial dysfunction, pioneering gut microorganisms, macrophage programming and epigenetic changes that alter the liver microenvironment, favouring liver injury. In this Review, we discuss how maternal, fetal, neonatal and infant exposures provide developmental clues and mechanisms to help explain NAFLD acceleration and increased disease prevalence. Mechanisms identified in clinical and preclinical models suggest important opportunities for prevention and intervention that could slow down the growing epidemic of NAFLD in the next generation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Welsh, J. A., Karpen, S. & Vos, M. B. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J. Pediatr. 162, 496–500 (2013).
Ogden, C. L. et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA 315, 2292–2299 (2016).
Armstrong, M. J., Adams, L. A., Canbay, A. & Syn, W. K. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology 59, 1174–1197 (2014).
Masuzaki, R., Karp, S. J. & Omata, M. NAFLD as a risk factor for HCC: new rules of engagement? Hepatol. Int. 10, 533–534 (2016).
Goyal, N. P. & Schwimmer, J. B. The progression and natural history of pediatric nonalcoholic fatty liver disease. Clin. Liver Dis. 20, 325–338 (2016).
Leung, J. C. et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology http://dx.doi.org/10.1002/hep.28697 (2016).
Wree, A., Broderick, L., Canbay, A., Hoffman, H. M. & Feldstein, A. E. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat. Rev. Gastroenterol. Hepatol. 10, 627–636 (2013).
Heerwagen, M. J., Miller, M. R., Barbour, L. A. & Friedman, J. E. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R711–R722 (2010).
Gluckman, P. D., Hanson, M. A., Cooper, C. & Thornburg, K. L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359, 61–73 (2008).
Weng, S. F. et al. Estimating overweight risk in childhood from predictors during infancy. Pediatrics 132, e414–e421 (2013).
Poston, L., Harthoorn, L. F. & Van Der Beek, E. M. Obesity in pregnancy: implications for the mother and lifelong health of the child. A consensus statement. Pediatr. Res. 69, 175–180 (2011).
Anderson, E. L. et al. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS ONE 10, e0140908 (2015).
Agopian, V. G. et al. Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann. Surg. 256, 624–633 (2012).
Gluckman, P. D. & Hanson, M. A. Living with the past: evolution, development, and patterns of disease. Science 305, 1733–1736 (2004).
Dabelea, D. & Crume, T. Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes 60, 1849–1855 (2011).
Boyle, K. E. et al. Mesenchymal stem cells from infants born to obese mothers exhibit greater potential for adipogenesis: the healthy start BabyBUMP project. Diabetes 65, 647–659 (2016).
Brumbaugh, D. E. et al. Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. J. Pediatr. 162, 930–936 (2013).
Modi, N. et al. The influence of maternal body mass index on infant adiposity and hepatic lipid content. Pediatr. Res. 70, 287–291 (2011).
Feldstein, A. E. et al. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut 58, 1538–1544 (2009).
Gale, C. et al. Adiposity and hepatic lipid in healthy full-term, breastfed, and formula-fed human infants: a prospective short-term longitudinal cohort study. Am. J. Clin. Nutr. 99, 1034–1040 (2014).
Patel, K. R., White, F. V. & Deutsch, G. H. Hepatic steatosis is prevalent in stillborns delivered to women with diabetes mellitus. J. Pediatr. Gastroenterol. Nutr. 60, 152–158 (2015).
Alisi, A., Panera, N., Agostoni, C. & Nobili, V. Intrauterine growth retardation and nonalcoholic fatty liver disease in children. Int. J. Endocrinol. 2011, 269853 (2011).
Nobili, V., Alisi, A., Panera, N. & Agostoni, C. Low birth weight and catch-up-growth associated with metabolic syndrome: a ten year systematic review. Pediatr. Endocrinol. Rev. 6, 241–247 (2008).
Baker, R. et al. Dysregulated lipid metabolism in adipocyte differentiated umbilical-derived mesenchymal stem cells predicts increased infant adiposity at 5 months of age. Diabetes 65 (Suppl. 1), A65 (2016).
Baker, R. et al. Metabolomic and proteomic analysis of neonatal plasma reveals novel evidence of early metabolic dysregulation from maternal obesity. Diabetes 65 (Suppl. 1), A8 (2016).
Stewart, M. S., Heerwagen, M. J. & Friedman, J. E. Developmental programming of pediatric nonalcoholic fatty liver disease: redefining the “first hit”. Clin. Obstet. Gynecol. 56, 577–590 (2013).
Heerwagen, M. J., Stewart, M. S., de la Houssaye, B. A., Janssen, R. C. & Friedman, J. E. Transgenic increase in n-3/n-6 fatty acid ratio reduces maternal obesity-associated inflammation and limits adverse developmental programming in mice. PLoS ONE 8, e67791 (2013).
McCurdy, C. E. et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J. Clin. Invest. 119, 323–335 (2009).
Thorn, S. R. et al. Early life exposure to maternal insulin resistance has persistent effects on hepatic NAFLD in juvenile nonhuman primates. Diabetes 63, 2702–2713 (2014).
Grant, W. F. et al. Maternal high fat diet is associated with decreased plasma n-3 fatty acids and fetal hepatic apoptosis in nonhuman primates. PLoS ONE 6, e17261 (2011).
Bayol, S. A., Simbi, B. H., Fowkes, R. C. & Stickland, N. C. A maternal “junk food” diet in pregnancy and lactation promotes nonalcoholic fatty liver disease in rat offspring. Endocrinology 151, 1451–1461 (2010).
Bruce, K. D. et al. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology 50, 1796–1808 (2009).
Mouralidarane, A. et al. Maternal obesity programs offspring nonalcoholic fatty liver disease by innate immune dysfunction in mice. Hepatology 58, 128–138 (2013).
Oben, J. A. et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J. Hepatol. 52, 913–920 (2010).
Wolfe, D. et al. Nutrient sensor-mediated programmed nonalcoholic fatty liver disease in low birthweight offspring. Am. J. Obstet. Gynecol. 207, 308.e1–308.e6 (2012).
Sarr, O. et al. The differential effects of low birth weight and Western diet consumption upon early life hepatic fibrosis development in guinea pig. J. Physiol. 594, 1753–1772 (2016).
Hyatt, M. A. et al. Suboptimal maternal nutrition, during early fetal liver development, promotes lipid accumulation in the liver of obese offspring. Reproduction 141, 119–126 (2011).
Pramfalk, C. et al. Sex-specific differences in hepatic fat oxidation and synthesis may explain the higher propensity for NAFLD in men. J. Clin. Endocrinol. Metab. 100, 4425–4433 (2015).
Strakovsky, R. S., Zhang, X., Zhou, D. & Pan, Y. X. The regulation of hepatic Pon1 by a maternal high-fat diet is gender specific and may occur through promoter histone modifications in neonatal rats. J. Nutr. Biochem. 25, 170–176 (2014).
Zhou, D., Wang, H., Cui, H., Chen, H. & Pan, Y. X. Early-life exposure to high-fat diet may predispose rats to gender-specific hepatic fat accumulation by programming Pepck expression. J. Nutr. Biochem. 26, 433–440 (2015).
Catalano, P. M. & Hauguel-De Mouzon, S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am. J. Obstet. Gynecol. 204, 479–487 (2011).
Schaefer-Graf, U. M. et al. Differences in the implications of maternal lipids on fetal metabolism and growth between gestational diabetes mellitus and control pregnancies. Diabet. Med. 28, 1053–1059 (2011).
Lawlor, D. A., Relton, C., Sattar, N. & Nelson, S. M. Maternal adiposity—a determinant of perinatal and offspring outcomes? Nat. Rev. Endocrinol. 8, 679–688 (2012).
Aye, I. L., Rosario, F. J., Powell, T. L. & Jansson, T. Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth. Proc. Natl. Acad. Sci. USA 112, 12858–12863 (2015).
Shapiro, A. L. et al. Testing the fuel-mediated hypothesis: maternal insulin resistance and glucose mediate the association between maternal and neonatal adiposity, the Healthy Start study. Diabetologia 58, 937–941 (2015).
Isganaitis, E. et al. Developmental programming by maternal insulin resistance: hyperinsulinemia, glucose intolerance, and dysregulated lipid metabolism in male offspring of insulin-resistant mice. Diabetes 63, 688–700 (2014).
Challier, J. C. et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 29, 274–281 (2008).
Dimasuay, K. G., Boeuf, P., Powell, T. L. & Jansson, T. Placental responses to changes in the maternal environment determine fetal growth. Front. Physiol. 7, 12 (2016).
Roberts, V. H. et al. Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J. 28, 2466–2477 (2014).
Frias, A. E. et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 152, 2456–2464 (2011).
Radaelli, T., Varastehpour, A., Catalano, P. & Hauguel-de Mouzon, S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes 52, 2951–2958 (2003).
Frias, A. E. & Grove, K. L. Obesity: a transgenerational problem linked to nutrition during pregnancy. Semin. Reprod. Med. 30, 472–478 (2012).
Li, H. P., Chen, X. & Li, M. Q. Gestational diabetes induces chronic hypoxia stress and excessive inflammatory response in murine placenta. Int. J. Clin. Exp. Pathol. 6, 650–659 (2013).
Aye, I. L., Jansson, T. & Powell, T. L. Interleukin-1β inhibits insulin signaling and prevents insulin-stimulated system A amino acid transport in primary human trophoblasts. Mol. Cell. Endocrinol. 381, 46–55 (2013).
Martin, U., Davies, C., Hayavi, S., Hartland, A. & Dunne, F. Is normal pregnancy atherogenic? Clin. Sci. (Lond.) 96, 421–425 (1999).
Montelongo, A., Lasunción, M. A., Pallardo, L. F. & Herrera, E. Longitudinal study of plasma lipoproteins and hormones during pregnancy in normal and diabetic women. Diabetes 41, 1651–1659 (1992).
Averna, M. R. et al. Lipids, lipoproteins and apolipoproteins AI, AII, B, CII, CIII and E in newborns. Biol. Neonate 60, 187–192 (1991).
Neary, R. H. et al. Fetal and maternal lipoprotein metabolism in human pregnancy. Clin. Sci. (Lond.) 88, 311–318 (1995).
McIntyre, H. D. et al. Hormonal and metabolic factors associated with variations in insulin sensitivity in human pregnancy. Diabetes Care 33, 356–360 (2010).
Radaelli, T. et al. Differential regulation of genes for fetoplacental lipid pathways in pregnancy with gestational and type 1 diabetes mellitus. Am. J. Obstet. Gynecol. 201, 209.e1–209.10 (2009).
Díaz, P., Harris, J., Rosario, F. J., Powell, T. L. & Jansson, T. Increased placental fatty acid transporter 6 and binding protein 3 expression and fetal liver lipid accumulation in a mouse model of obesity in pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R1569–R1577 (2015).
López-Luna, P., Ortega-Senovilla, H., López-Soldado, I. & Herrera, E. Fate of orally administered radioactive fatty acids in the late-pregnant rat. Am. J. Physiol. Endocrinol. Metab. 310, E367–E377 (2016).
Lager, S., Jansson, T. & Powell, T. L. Differential regulation of placental amino acid transport by saturated and unsaturated fatty acids. Am. J. Physiol. Cell Physiol. 307, C738–C744 (2014).
Lager, S. et al. Oleic acid stimulates system A amino acid transport in primary human trophoblast cells mediated by toll-like receptor 4. J. Lipid Res. 54, 725–733 (2013).
Beijar, E. C., Mallard, C. & Powell, T. L. Expression and subcellular localization of TLR-4 in term and first trimester human placenta. Placenta 27, 322–326 (2006).
Musso, G. et al. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes. Rev. 14, 417–431 (2013).
Taricco, E. et al. Effects of gestational diabetes on fetal oxygen and glucose levels in vivo. BJOG 116, 1729–1735 (2009).
Hayes, E. K. et al. Adverse fetal and neonatal outcomes associated with a life-long high fat diet: role of altered development of the placental vasculature. PLoS ONE 7, e33370 (2012).
Higgins, M., Felle, P., Mooney, E. E., Bannigan, J. & McAuliffe, F. M. Stereology of the placenta in type 1 and type 2 diabetes. Placenta 32, 564–569 (2011).
Li, Y. et al. GDM-associated insulin deficiency hinders the dissociation of SERT from ERp44 and down-regulates placental 5-HT uptake. Proc. Natl. Acad. Sci. USA 111, E5697–E5705 (2014).
Cao, L. et al. Hepatic insulin signaling changes: possible mechanism in prenatal hypoxia-increased susceptibility of fatty liver in adulthood. Endocrinology 153, 4955–4965 (2012).
Sun, Z. & Lazar, M. A. Dissociating fatty liver and diabetes. Trends Endocrinol. Metab. 24, 4–12 (2013).
Hashimoto, K. et al. Protective effect of N-acetylcysteine on liver damage during chronic intrauterine hypoxia in fetal guinea pig. Reprod. Sci. 19, 1001–1009 (2012).
Magee, T. R. et al. Down-regulation of transcription factor peroxisome proliferator-activated receptor in programmed hepatic lipid dysregulation and inflammation in intrauterine growth-restricted offspring. Am. J. Obstet. Gynecol. 199, 271.e1–271.e5 (2008).
Rueda-Clausen, C. F. et al. Hypoxia-induced intrauterine growth restriction increases the susceptibility of rats to high-fat diet-induced metabolic syndrome. Diabetes 60, 507–516 (2011).
Bell, R. M. & Coleman, R. A. Enzymes of glycerolipid synthesis in eukaryotes. Annu. Rev. Biochem. 49, 459–487 (1980).
Suter, M. A. et al. A maternal high-fat diet modulates fetal SIRT1 histone and protein deacetylase activity in nonhuman primates. FASEB J. 26, 5106–5114 (2012).
Bernstein, I. M., Goran, M. I., Amini, S. B. & Catalano, P. M. Differential growth of fetal tissues during the second half of pregnancy. Am. J. Obstet. Gynecol. 176, 28–32 (1997).
Satapati, S. et al. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J. Lipid Res. 53, 1080–1092 (2012).
Sunny, N. E., Parks, E. J., Browning, J. D. & Burgess, S. C. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 14, 804–810 (2011).
Koliaki, C. & Roden, M. Hepatic energy metabolism in human diabetes mellitus, obesity and non-alcoholic fatty liver disease. Mol. Cell. Endocrinol. 379, 35–42 (2013).
Alkhouri, N., Carter-Kent, C. & Feldstein, A. E. Apoptosis in nonalcoholic fatty liver disease: diagnostic and therapeutic implications. Expert Rev. Gastroenterol. Hepatol. 5, 201–212 (2011).
Thompson, L. P. & Al-Hasan, Y. Impact of oxidative stress in fetal programming. J. Pregnancy 2012, 582748 (2012).
McCoin, C. S., Knotts, T. A., Ono-Moore, K. D., Oort, P. J. & Adams, S. H. Long-chain acylcarnitines activate cell stress and myokine release in C2C12 myotubes: calcium-dependent and -independent effects. Am. J. Physiol. Endocrinol. Metab. 308, E990–E1000 (2015).
Kaochar, S. & Tu, B. P. Gatekeepers of chromatin: small metabolites elicit big changes in gene expression. Trends Biochem. Sci. 37, 477–483 (2012).
McCurdy, C. E. et al. Maternal obesity reduces fetal skeletal muscle oxidative capacity in Japanese macaques. JCI Insight, 1, e86612 (2016).
Borengasser, S. J. et al. Maternal obesity during gestation impairs fatty acid oxidation and mitochondrial SIRT3 expression in rat offspring at weaning. PLoS ONE 6, e24068 (2011).
Burgueño, A. L., Cabrerizo, R., Gonzales Mansilla, N., Sookoian, S. & Pirola, C. J. Maternal high-fat intake during pregnancy programs metabolic-syndrome-related phenotypes through liver mitochondrial DNA copy number and transcriptional activity of liver PPARGC1A. J. Nutr. Biochem. 24, 6–13 (2013).
Sookoian, S. et al. Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: impact of liver methylation of the peroxisome proliferator-activated receptor γ coactivator 1α promoter. Hepatology 52, 1992–2000 (2010).
Gallego-Durán, R. & Romero-Gómez, M. Epigenetic mechanisms in non-alcoholic fatty liver disease: an emerging field. World J. Hepatol. 7, 2497–2502 (2015).
Sookoian, S., Gianotti, T. F., Burgueño, A. L. & Pirola, C. J. Fetal metabolic programming and epigenetic modifications: a systems biology approach. Pediatr. Res. 73, 531–542 (2013).
Suter, M. A., Takahashi, D., Grove, K. L. & Aagaard, K. M. Postweaning exposure to a high-fat diet is associated with alterations to the hepatic histone code in Japanese macaques. Pediatr. Res. 74, 252–258 (2013).
Gemma, C. et al. Maternal pregestational BMI is associated with methylation of the PPARGC1A promoter in newborns. Obesity (Silver Spring) 17, 1032–1039 (2009).
Igosheva, N. et al. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS ONE 5, e10074 (2010).
Jungheim, E. S. et al. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology 151, 4039–4046 (2010).
Bäckhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 101, 15718–15723 (2004).
Harley, I. T. et al. IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology 59, 1830–1839 (2014).
Boursier, J. et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 63, 764–775 (2016).
Wieland, A., Frank, D. N., Harnke, B. & Bambha, K. Systematic review: microbial dysbiosis and nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 42, 1051–1063 (2015).
Schnabl, B. & Brenner, D. A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 146, 1513–1524 (2014).
Singh, V. et al. Microbiota-dependent hepatic lipogenesis mediated by stearoyl CoA desaturase 1 (SCD1) promotes metabolic syndrome in TLR5-deficient mice. Cell Metab. 22, 983–996 (2015).
Ridlon, J. M., Kang, D. J., Hylemon, P. B. & Bajaj, J. S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 30, 332–338 (2014).
den Besten, G. et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340 (2013).
Leung, C., Rivera, L., Furness, J. B. & Angus, P. W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 13, 412–425 (2016).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Walker, A. W. et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 5, 220–230 (2011).
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA 107, 14691–14696 (2010).
Ley, R. E. et al. Evolution of mammals and their gut microbes. Science 320, 1647–1651 (2008).
Muegge, B. D. et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 332, 970–974 (2011).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Wu, G. D. et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 65, 63–72 (2016).
Ma, J. et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat. Commun. 5, 3889 (2014).
Chu, D. M. et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 8, 77 (2016).
Myles, I. A. et al. Parental dietary fat intake alters offspring microbiome and immunity. J. Immunol. 191, 3200–3209 (2013).
Collado, M. C., Isolauri, E., Laitinen, K. & Salminen, S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 88, 894–899 (2008).
Santacruz, A. et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 104, 83–92 (2010).
Kalliomäki, M., Collado, M. C., Salminen, S. & Isolauri, E. Early differences in fecal microbiota composition in children may predict overweight. Am. J. Clin. Nutr. 87, 534–538 (2008).
Soderborg, T. K., Borengasser, S. J., Barbour, L. A. & Friedman, J. E. Microbial transmission from mothers with obesity or diabetes to infants: an innovative opportunity to interrupt a vicious cycle. Diabetologia 59, 895–906 (2016).
Donnet-Hughes, A. et al. Potential role of the intestinal microbiota of the mother in neonatal immune education. Proc. Nutr. Soc. 69, 407–415 (2010).
Van den Abbeele, P., Van de Wiele, T., Verstraete, W. & Possemiers, S. The host selects mucosal and luminal associations of coevolved gut microorganisms: a novel concept. FEMS Microbiol. Rev. 35, 681–704 (2011).
Maynard, C. L., Elson, C. O., Hatton, R. D. & Weaver, C. T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 489, 231–241 (2012).
Khodayar-Pardo, P., Mira-Pascual, L., Collado, M. C. & Martinez-Costa, C. Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J. Perinatol. 34, 599–605 (2014).
Cabrera-Rubio, R. et al. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am. J. Clin. Nutr. 96, 544–551 (2012).
Latuga, M. S., Stuebe, A. & Seed, P. C. A review of the source and function of microbiota in breast milk. Semin. Reprod. Med. 32, 68–73 (2014).
Hunt, K. M. et al. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE 6, e21313 (2011).
Martín, R., Heilig, G. H., Zoetendal, E. G., Smidt, H. & Rodríguez, J. M. Diversity of the Lactobacillus group in breast milk and vagina of healthy women and potential role in the colonization of the infant gut. J. Appl. Microbiol. 103, 2638–2644 (2007).
Solís, G., de Los Reyes-Gavilan, C. G., Fernández, N., Margolles, A. & Gueimonde, M. Establishment and development of lactic acid bacteria and bifidobacteria microbiota in breast-milk and the infant gut. Anaerobe 16, 307–310 (2010).
Coppa, G. V., Bruni, S., Morelli, L., Soldi, S. & Gabrielli, O. The first prebiotics in humans: human milk oligosaccharides. J. Clin. Gastroenterol. 38, S80–S83 (2004).
Bode, L. Human milk oligosaccharides: prebiotics and beyond. Nutr. Rev. 67, S183–S191 (2009).
Azad, M. B. et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ 185, 385–394 (2013).
Houghteling, P. D. & Walker, W. A. Why is initial bacterial colonization of the intestine important to infants' and children's health? J. Pediatr. Gastroenterol. Nutr. 60, 294–307 (2015).
Giannone, P. J., Schanbacher, B. L., Bauer, J. A. & Reber, K. M. Effects of prenatal lipopolysaccharide exposure on epithelial development and function in newborn rat intestine. J. Pediatr. Gastroenterol. Nutr. 43, 284–290 (2006).
Mirpuri, J. et al. Proteobacteria-specific IgA regulates maturation of the intestinal microbiota. Gut Microbes 5, 28–39 (2014).
Wang, Y. & McCusker, C. Neonatal exposure with LPS and/or allergen prevents experimental allergic airways disease: development of tolerance using environmental antigens. J. Allergy Clin. Immunol. 118, 143–151 (2006).
Lemas, D. J. et al. Alterations in human milk leptin and insulin are associated with early changes in the infant intestinal microbiome. Am. J. Clin. Nutr. 103, 1291–1300 (2016).
Fu, S., Watkins, S. M. & Hotamisligil, G. S. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 15, 623–634 (2012).
Henkel, A. & Green, R. M. The unfolded protein response in fatty liver disease. Semin. Liver Dis. 33, 321–329 (2013).
Puri, P. et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 134, 568–576 (2008).
Gregor, M. F. et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 58, 693–700 (2009).
Arab, J. P., Karpen, S. J., Dawson, P. A., Arrese, M. & Trauner, M. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology http://dx.doi.org/10.1002/hep.28709 (2016).
Ridlon, J. M. & Bajaj, J. S. The human gut sterolbiome: bile acid-microbiome endocrine aspects and therapeutics. Acta Pharm. Sin. B 5, 99–105 (2015).
Inagaki, T. et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc. Natl. Acad. Sci. USA 103, 3920–3925 (2006).
Studer, E. et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology 55, 267–276 (2012).
Hylemon, P. B. et al. Bile acids as regulatory molecules. J. Lipid Res. 50, 1509–1520 (2009).
Kawamata, Y. et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 278, 9435–9440 (2003).
Seok, S. et al. Transcriptional regulation of autophagy by an FXR-CREB axis. Nature 516, 108–111 (2014).
Perino, A. & Schoonjans, K. TGR5 and immunometabolism: insights from physiology and pharmacology. Trends Pharmacol. Sci. 36, 847–857 (2015).
Yao, J. et al. FXR agonist GW4064 alleviates endotoxin-induced hepatic inflammation by repressing macrophage activation. World J. Gastroenterol. 20, 14430–14441 (2014).
Sayin, S. I. et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-β-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17, 225–235 (2013).
Papacleovoulou, G. et al. Maternal cholestasis during pregnancy programs metabolic disease in offspring. J. Clin. Invest. 123, 3172–3181 (2013).
Fiorucci, S. & Distrutti, E. Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders. Trends Mol. Med. 21, 702–714 (2015).
McMahan, R. H. et al. Bile acid receptor activation modulates hepatic monocyte activity and improves nonalcoholic fatty liver disease. J. Biol. Chem. 288, 11761–11770 (2013).
Calmus, Y. & Poupon, R. Shaping macrophages function and innate immunity by bile acids: mechanisms and implication in cholestatic liver diseases. Clin. Res. Hepatol. Gastroenterol. 38, 550–556 (2014).
Koenig, J. E. et al. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl Acad. Sci. USA 108, 4578–4585 (2011).
Bäckhed, F. et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703 (2015).
Giorgio, V. et al. Intestinal permeability is increased in children with non-alcoholic fatty liver disease, and correlates with liver disease severity. Dig. Liver Dis. 46, 556–560 (2014).
Weaver, L. T., Laker, M. F. & Nelson, R. Intestinal permeability in the newborn. Arch. Dis. Child 59, 236–241 (1984).
Jakobsdottir, G., Xu, J., Molin, G., Ahrné, S. & Nyman, M. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS ONE 8, e80476 (2013).
Dawiskiba, T. et al. Serum and urine metabolomic fingerprinting in diagnostics of inflammatory bowel diseases. World J. Gastroenterol. 20, 163–174 (2014).
Kotas, M. E. & Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 160, 816–827 (2015).
Okabe, Y. & Medzhitov, R. Tissue biology perspective on macrophages. Nat. Immunol. 17, 9–17 (2016).
Wynn, T. A., Chawla, A. & Pollard, J. W. Macrophage biology in development, homeostasis and disease. Nature 496, 445–455 (2013).
Okabe, Y. & Medzhitov, R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell 157, 832–844 (2014).
Xue, J. et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288 (2014).
Duffield, J. S. et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 115, 56–65 (2005).
Ramachandran, P. et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl. Acad. Sci. USA 109, E3186–E3195 (2012).
Tacke, F. & Zimmermann, H. W. Macrophage heterogeneity in liver injury and fibrosis. J. Hepatol. 60, 1090–1096 (2014).
Karlmark, K. R. et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 50, 261–274 (2009).
Karlmark, K. R. et al. The fractalkine receptor CX3CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology 52, 1769–1782 (2010).
Seki, E. et al. CCR2 promotes hepatic fibrosis in mice. Hepatology 50, 185–197 (2009).
Ensan, S. et al. Self-renewing resident arterial macrophages arise from embryonic CX3CR1+ precursors and circulating monocytes immediately after birth. Nat. Immunol. 17, 159–168 (2016).
Brenner, D. A., Paik, Y. H. & Schnabl, B. Role of gut microbiota in liver disease. J. Clin. Gastroenterol. 49 (Suppl. 1), S25–S27 (2015).
Ganz, M. & Szabo, G. Immune and inflammatory pathways in NASH. Hepatol. Int. 7 (Suppl. 2), 771–781 (2013).
Szabo, G., Bala, S., Petrasek, J. & Gattu, A. Gut-liver axis and sensing microbes. Dig. Dis. 28, 737–744 (2010).
Ilan, Y. Leaky gut and the liver: a role for bacterial translocation in nonalcoholic steatohepatitis. World J. Gastroenterol. 18, 2609–2618 (2012).
Mazagova, M. et al. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB J. 29, 1043–1055 (2015).
Hartmann, P., Haimerl, M., Mazagova, M., Brenner, D. A. & Schnabl, B. Toll-like receptor 2-mediated intestinal injury and enteric tumor necrosis factor receptor I contribute to liver fibrosis in mice. Gastroenterology 143, 1330–1340 (2012).
Fouts, D. E., Torralba, M., Nelson, K. E., Brenner, D. A. & Schnabl, B. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J. Hepatol. 56, 1283–1292 (2012).
Miura, K., Seki, E., Ohnishi, H. & Brenner, D. A. Role of toll-like receptors and their downstream molecules in the development of nonalcoholic fatty liver disease. Gastroenterol. Res. Pract. 2010, 362847 (2010).
Seki, E. et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat. Med. 13, 1324–1332 (2007).
El Kasmi, K. C. et al. Toll-like receptor 4-dependent Kupffer cell activation and liver injury in a novel mouse model of parenteral nutrition and intestinal injury. Hepatology 55, 1518–1528 (2012).
Tilg, H., Moschen, A. R. & Szabo, G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 64, 955–965 (2016).
Szabo, G. Gut-liver axis in alcoholic liver disease. Gastroenterology 148, 30–36 (2015).
Chen, P., Starkel, P., Turner, J. R., Ho, S. B. & Schnabl, B. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology 61, 883–894 (2015).
Chen, P. et al. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology 148, 203–214 (2015).
Schnabl, B. Linking intestinal homeostasis and liver disease. Curr. Opin. Gastroenterol. 29, 264–270 (2013).
Miura, K. et al. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology 57, 577–589 (2013).
Iraporda, C. et al. Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology 220, 1161–1169 (2015).
Österreicher, C. H. et al. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc. Natl Acad. Sci. USA 108, 308–313 (2011).
El Kasmi, K. C. & Stenmark, K. R. Contribution of metabolic reprogramming to macrophage plasticity and function. Semin. Immunol. 27, 267–275 (2015).
Ramachandran, P., Iredale, J. P. & Fallowfield, J. A. Resolution of liver fibrosis: basic mechanisms and clinical relevance. Semin. Liver Dis. 35, 119–131 (2015).
Pellicoro, A., Ramachandran, P., Iredale, J. P. & Fallowfield, J. A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 14, 181–194 (2014).
El Kasmi, K. C. et al. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J. Immunol. 193, 597–609 (2014).
Netea, M. G. et al. Trained immunity: a program of innate immune memory in health and disease. Science 352, http://dx.doi.org/10.1126/science.aaf1098 (2016).
Saeed, S. et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345, http://dx.doi.org/10.1126/science.1251086 (2014).
Cheng, S. C. et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345, http://dx.doi.org/10.1126/science.1250684 (2014).
O'Neill, L. A. & Pearce, E. J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 213, 15–23 (2016).
Foster, S. L., Hargreaves, D. C. & Medzhitov, R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447, 972–978 (2007).
Mills, E. L. & O'Neill, L. A. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur. J. Immunol. 46, 13–21 (2016).
Ng, R. L. et al. Altered immunity and dendritic cell activity in the periphery of mice after long-term engraftment with bone marrow from ultraviolet-irradiated mice. J. Immunol. 190, 5471–5484 (2013).
Burgess, S. L. et al. Bone marrow dendritic cells from mice with an altered microbiota provide interleukin 17A-dependent protection against Entamoeba histolytica colitis. MBio 5, e01817 (2014).
Galván-Peña, S. & O'Neill, L. A. Metabolic reprograming in macrophage polarization. Front. Immunol. 5, 420 (2014).
Palsson-McDermott, E. M. et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 21, 65–80 (2015).
Perdiguero, E. G. & Geissmann, F. The development and maintenance of resident macrophages. Nat. Immunol. 17, 2–8 (2016).
Kierdorf, K., Prinz, M., Geissmann, F. & Gomez Perdiguero, E. Development and function of tissue resident macrophages in mice. Semin. Immunol. 27, 369–378 (2016).
Hoeffel, G. et al. C-Myb+ erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42, 665–678 (2015).
Piccinini, A. M., Zuliani-Alvarez, L., Lim, J. M. P. & Midwood, K. S. Distinct microenvironmental cues stimulate divergent TLR4-mediated signaling pathways in macrophages. Sci. Signal. 9, ra86 (2016).
Harris, J. K. et al. Specific microbiome changes in a mouse model of parenteral nutrition associated liver injury and intestinal inflammation. PLoS ONE 9, e110396 (2014).
El Kasmi, K. C. et al. Phytosterols promote liver injury and Kupffer cell activation in parenteral nutrition-associated liver disease. Sci. Transl. Med. 5, 206ra137 (2013).
Morris, M. W. Jr et al. Modulation of the inflammatory response by increasing fetal wound size or interleukin-10 overexpression determines wound phenotype and scar formation. Wound Repair Regen. 22, 406–414 (2014).
Godfrey, K. M., Gluckman, P. D. & Hanson, M. A. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol. Metab. 21, 199–205 (2010).
Syngelaki, A. et al. Metformin versus placebo in obese pregnant women without diabetes mellitus. N. Engl. J. Med. 374, 434–443 (2016).
Chiswick, C. et al. Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 3, 778–786 (2015).
Rowan, J. A., Hague, W. M., Gao, W., Battin, M. R. & Moore, M. P. Metformin versus insulin for the treatment of gestational diabetes. N. Engl. J. Med. 358, 2003–2015 (2008).
Rowan, J. A. et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition at 2 years of age. Diabetes Care 34, 2279–2284 (2011).
Flynn, A. C. et al. Dietary interventions in overweight and obese pregnant women: a systematic review of the content, delivery, and outcomes of randomized controlled trials. Nutr. Rev. 74, 312–328 (2016).
Oteng-Ntim, E., Varma, R., Croker, H., Poston, L. & Doyle, P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta-analysis. BMC Med. 10, 47 (2012).
Hernandez, T. L. et al. Women with gestational diabetes randomized to a higher-complex carbohydrate/low-fat diet manifest lower adipose tissue insulin resistance, inflammation, glucose, and free fatty acids: a pilot study. Diabetes Care 39, 39–42 (2015).
Hernandez, T. L. et al. A higher-complex carbohydrate diet in gestational diabetes mellitus achieves glucose targets and lowers postprandial lipids: a randomized crossover study. Diabetes Care 37, 1254–1262 (2014).
Patterson, E. et al. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 92, 286–300 (2016).
Cotillard, A. et al. Dietary intervention impact on gut microbial gene richness. Nature 500, 585–588 (2013).
Le Chatelier, E. et al. Richness of human gut microbiome correlates with metabolic markers. Nature 500, 541–546 (2013).
Remely, M. et al. Gut microbiota composition correlates with changes in body fat content due to weight loss. Benef. Microbes 6, 431–439 (2015).
Rautava, S., Collado, M. C., Salminen, S. & Isolauri, E. Probiotics modulate host-microbe interaction in the placenta and fetal gut: a randomized, double-blind, placebo-controlled trial. Neonatology 102, 178–184 (2012).
Ilmonen, J., Isolauri, E., Poussa, T. & Laitinen, K. Impact of dietary counselling and probiotic intervention on maternal anthropometric measurements during and after pregnancy: a randomized placebo-controlled trial. Clin. Nutr. 30, 156–164 (2011).
Luoto, R., Kalliomäki, M., Laitinen, K. & Isolauri, E. The impact of perinatal probiotic intervention on the development of overweight and obesity: follow-up study from birth to 10 years. Int. J. Obes. (Lond.) 34, 1531–1537 (2010).
Vrieze, A. et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916 (2012).
Savcheniuk, O. et al. Short-term periodic consumption of multiprobiotic from childhood improves insulin sensitivity, prevents development of non-alcoholic fatty liver disease and adiposity in adult rats with glutamate-induced obesity. BMC Complement. Altern. Med. 14, 247 (2014).
Singh, A. K., Pandey, S. K. & Naresh Kumar, G. Pyrroloquinoline quinone-secreting probiotic Escherichia coli Nissle 1917 ameliorates ethanol-induced oxidative damage and hyperlipidemia in rats. Alcohol. Clin. Exp. Res. 38, 2127–2137 (2014).
Pham, V. T., Lacroix, C., Braegger, C. P. & Chassard, C. Early colonization of functional groups of microbes in the infant gut. Environ. Microbiol. 18, 2246–2258 (2016).
Mudaliar, S. et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 145, 574–582 (2013).
Neuschwander-Tetri, B. A. et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385, 956–965 (2015).
Carr, R. M. & Reid, A. E. FXR agonists as therapeutic agents for non-alcoholic fatty liver disease. Curr. Atheroscler. Rep. 17, http://dx.doi.org/10.1007/s11883-015-0500-2 (2015).
Ferslew, B. C. et al. Altered bile acid metabolome in patients with nonalcoholic steatohepatitis. Dig. Dis. Sci. 60, 3318–3328 (2015).
Bernhard, W. et al. Choline supply of preterm infants: assessment of dietary intake and pathophysiological considerations. Eur. J. Nutr. 52, 1269–1278 (2013).
Cai, D. et al. Maternal dietary betaine supplementation modifies hepatic expression of cholesterol metabolic genes via epigenetic mechanisms in newborn piglets. Br. J. Nutr. 112, 1459–1468 (2014).
Guy, C. D. et al. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology 55, 1711–1721 (2012).
Briscoe, J. & Thérond, P. P. The mechanisms of hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 14, 416–429 (2013).
Kwon, H. et al. Inhibition of hedgehog signaling ameliorates hepatic inflammation in mice with nonalcoholic fatty liver disease. Hepatology 63, 1155–1169 (2016).
Guy, C. D., Suzuki, A., Abdelmalek, M. F., Burchette, J. L. & Diehl, A. M. Treatment response in the PIVENS trial is associated with decreased hedgehog pathway activity. Hepatology 61, 98–107 (2015).
West, C. E. et al. Associations between maternal antioxidant intakes in pregnancy and infant allergic outcomes. Nutrients 4, 1747–1758 (2012).
Greenough, A., Shaheen, S. O., Shennan, A., Seed, P. T. & Poston, L. Respiratory outcomes in early childhood following antenatal vitamin C and E supplementation. Thorax 65, 998–1003 (2010).
Infante, P., Alfonsi, R., Botta, B., Mori, M. & Di Marcotullio, L. Targeting GLI factors to inhibit the hedgehog pathway. Trends Pharmacol. Sci. 36, 547–558 (2015).
Magnusardottir, A. R., Steingrimsdottir, L., Thorgeirsdottir, H., Hauksson, A. & Skuladottir, G. V. Red blood cell n-3 polyunsaturated fatty acids in first trimester of pregnancy are inversely associated with placental weight. Acta Obstet. Gynecol. Scand. 88, 91–97 (2009).
Stene, L. C. & Joner, G. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case-control study. Am. J. Clin. Nutr. 78, 1128–1134 (2003).
Oien, T., Storrø, O. & Johnsen, R. Do early intake of fish and fish oil protect against eczema and doctor-diagnosed asthma at 2 years of age? A cohort study. J. Epidemiol. Community Health 64, 124–129 (2010).
Loguercio, C. et al. Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Free Radic. Biol. Med. 52, 1658–1665 (2012).
Sorrentino, G., Crispino, P., Coppola, D. & De Stefano, G. Efficacy of lifestyle changes in subjects with non-alcoholic liver steatosis and metabolic syndrome may be improved with an antioxidant nutraceutical: a controlled clinical study. Drugs R. D. 15, 21–25 (2015).
Sen, S. & Simmons, R. A. Maternal antioxidant supplementation prevents adiposity in the offspring of Western diet-fed rats. Diabetes 59, 3058–3065 (2010).
Chen, S. et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Dig. Liver Dis. 47, 226–232 (2015).
Vega, C. C. et al. Resveratrol partially prevents oxidative stress and metabolic dysfunction in pregnant rats fed a low protein diet and their offspring. J. Physiol. 594, 1483–1499 (2016).
Bourque, S. L., Dolinsky, V. W., Dyck, J. R. & Davidge, S. T. Maternal resveratrol treatment during pregnancy improves adverse fetal outcomes in a rat model of severe hypoxia. Placenta 33, 449–452 (2012).
Poudel, R. et al. Effects of resveratrol in pregnancy using murine models with reduced blood supply to the uterus. PLoS ONE 8, e64401 (2013).
Hassanzadeh, P., Arbabi, E., Atyabi, F. & Dinarvand, R. The endocannabinoid system and NGF are involved in the mechanism of action of resveratrol: a multi-target nutraceutical with therapeutic potential in neuropsychiatric disorders. Psychopharmacology (Berl.) 233, 1087–1096 (2016).
Pacher, P. & Kunos, G. Modulating the endocannabinoid system in human health and disease—successes and failures. FEBS J. 280, 1918–1943 (2013).
Maccarrone, M. et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 36, 277–296 (2015).
Lipina, C., Rastedt, W., Irving, A. J. & Hundal, H. S. New vistas for treatment of obesity and diabetes? Endocannabinoid signalling and metabolism in the modulation of energy balance. Bioessays 34, 681–691 (2012).
Fezza, F. et al. Endocannabinoids, related compounds and their metabolic routes. Molecules 19, 17078–17106 (2014).
Pertwee, R. G. et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2 . Pharmacol. Rev. 62, 588–631 (2010).
Cani, P. D., Everard, A. & Duparc, T. Gut microbiota, enteroendocrine functions and metabolism. Curr. Opin. Pharmacol. 13, 935–940 (2013).
Jourdan, T. et al. Antagonism of peripheral hepatic cannabinoid receptor-1 improves liver lipid metabolism in mice: evidence from cultured explants. Hepatology 55, 790–799 (2012).
Liu, J. et al. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology 142, 1218–1228 (2012).
Jeong, W. I. et al. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab. 7, 227–235 (2008).
Julien, B. et al. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 128, 742–755 (2005).
Mallat, A., Teixeira-Clerc, F., Deveaux, V., Manin, S. & Lotersztajn, S. The endocannabinoid system as a key mediator during liver diseases: new insights and therapeutic openings. Br. J. Pharmacol. 163, 1432–1440 (2011).
Muñoz-Luque, J. et al. Regression of fibrosis after chronic stimulation of cannabinoid CB2 receptor in cirrhotic rats. J. Pharmacol. Exp. Ther. 324, 475–483 (2008).
Guillot, A. et al. Cannabinoid receptor 2 counteracts interleukin-17-induced immune and fibrogenic responses in mouse liver. Hepatology 59, 296–306 (2014).
Louvet, A. et al. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology 54, 1217–1226 (2011).
Bátkai, S. et al. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J. 21, 1788–1800 (2007).
Cao, Z. et al. Monoacylglycerol lipase controls endocannabinoid and eicosanoid signaling and hepatic injury in mice. Gastroenterology 144, 808–817 (2013).
Tanvig, M. et al. Anthropometrics and body composition by dual energy X-ray in children of obese women: a follow-up of a randomized controlled trial (the Lifestyle in Pregnancy and Offspring [LiPO] study). PLoS ONE 9, e89590 (2014).
Gillman, M. W. et al. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care 33, 964–968 (2010).
Martin, R. M. et al. Effects of promoting longer-term and exclusive breastfeeding on cardiometabolic risk factors at age 11.5 years: a cluster-randomized, controlled trial. Circulation 129, 321–329 (2014).
Kramer, M. S. et al. Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: evidence from a large randomized trial. Am. J. Clin. Nutr. 86, 1717–1721 (2007).
Wen, L. M. et al. Effectiveness of home based early intervention on children's BMI at age 2: randomised controlled trial. BMJ 344, e3732 (2012).
Navarro, J. I., Sigulem, D. M., Ferraro, A. A., Polanco, J. J. & Barros, A. J. The double task of preventing malnutrition and overweight: a quasi-experimental community-based trial. BMC Public Health 13, 212 (2013).
Hauner, H. et al. Effect of reducing the n-6:n-3 long-chain PUFA ratio during pregnancy and lactation on infant adipose tissue growth within the first year of life: an open-label randomized controlled trial. Am. J. Clin. Nutr. 95, 383–394 (2012).
Much, D. et al. Effect of dietary intervention to reduce the n-6/n-3 fatty acid ratio on maternal and fetal fatty acid profile and its relation to offspring growth and body composition at 1 year of age. Eur. J. Clin. Nutr. 67, 282–288 (2013).
Lauritzen, L., Hoppe, C., Straarup, E. M. & Michaelsen, K. F. Maternal fish oil supplementation in lactation and growth during the first 2.5 years of life. Pediatr. Res. 58, 235–242 (2005).
Asserhøj, M., Nehammer, S., Matthiessen, J., Michaelsen, K. F. & Lauritzen, L. Maternal fish oil supplementation during lactation may adversely affect long-term blood pressure, energy intake, and physical activity of 7-year-old boys. J. Nutr. 139, 298–304 (2009).
Andersen, A. D., Michaelsen, K. F., Hellgren, L. I., Trolle, E. & Lauritzen, L. A randomized controlled intervention with fish oil versus sunflower oil from 9 to 18 months of age: exploring changes in growth and skinfold thicknesses. Pediatr. Res. 70, 368–374 (2011).
Acknowledgements
The authors are supported by the NIH–National Institute of Diabetes and Digestive and Kidney Diseases (R24 DK090964), the American Diabetes Association (ADA 1-13-GSK-13), and the Colorado NIH Nutrition and Obesity Research Center (NORC; P30 DK048520). We thank R. C. Janssen for help with editing the manuscript.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, provided a substantial contribution to discussion of content, wrote the article, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wesolowski, S., Kasmi, K., Jonscher, K. et al. Developmental origins of NAFLD: a womb with a clue. Nat Rev Gastroenterol Hepatol 14, 81–96 (2017). https://doi.org/10.1038/nrgastro.2016.160
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2016.160
This article is cited by
-
High-fat diet in early life triggers both reversible and persistent epigenetic changes in the medaka fish (Oryzias latipes)
BMC Genomics (2023)
-
Exploring immune related gene signatures and mechanisms linking non alcoholic fatty liver disease to atrial fibrillation through transcriptome data analysis
Scientific Reports (2023)
-
Hepatocyte FBXW7-dependent activity of nutrient-sensing nuclear receptors controls systemic energy homeostasis and NASH progression in male mice
Nature Communications (2023)
-
Prevalence and incidence of MAFLD and associated anthropometric parameters among prepubertal children of the Shanghai Birth Cohort
Hepatology International (2023)
-
Gender differences in the ideal cutoffs of visceral fat area for predicting MAFLD in China
Lipids in Health and Disease (2022)