Key Points
-
Many oncogenic signalling pathways converge on signal transducer and activator of transcription 3 (STAT3). As a result, STAT3 is constitutively activated in a range of cancers.
-
STAT3 is a broad transcriptional regulator, and constitutively active STAT3 leads to a gene-expression pattern that promotes tumour-cell survival and proliferation, tumour angiogenesis and metastasis.
-
STAT3 provides the first direct link between oncogenesis and immune evasion. STAT3 activity inhibits the expression of T helper 1 (TH1)-type immunostimulating molecules while promoting the expression of immunosuppressive factors.
-
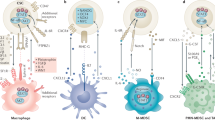
STAT3 activity propagates from tumour cells to immune cells in the tumour microenvironment, thereby mediating immune evasion by blocking both the production and sensing of inflammatory signals by various components of the immune system.
-
The propagation of STAT3 activity from tumour cells to diverse immune cells, and from one immune cell type to another, and back to tumour cells, is accomplished by STAT3-regulated factors, such as interleukin-10 and vascular endothelial growth factor, that are also STAT3 activators.
-
Targeting STAT3 in either tumour cells or immune cells stimulates both innate and adaptive immune responses against the tumour.
-
Combining STAT3 targeting with other promising immunotherapeutic approach(es) is anticipated to generate optimal anti-tumour immune responses.
Abstract
Immune cells in the tumour microenvironment not only fail to mount an effective anti-tumour immune response, but also interact intimately with the transformed cells to promote oncogenesis actively. Signal transducer and activator of transcription 3 (STAT3), which is a point of convergence for numerous oncogenic signalling pathways, is constitutively activated both in tumour cells and in immune cells in the tumour microenvironment. Constitutively activated STAT3 inhibits the expression of mediators necessary for immune activation against tumour cells. Furthermore, STAT3 activity promotes the production of immunosuppressive factors that activate STAT3 in diverse immune-cell subsets, altering gene-expression programmes and, thereby, restraining anti-tumour immune responses. As such, STAT3 propagates several levels of crosstalk between tumour cells and their immunological microenvironment, leading to tumour-induced immunosuppression. Consequently, STAT3 has emerged as a promising target for cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Bishop, J. M. Cancer: what should be done? Science 278, 995 (1997).
Vogelstein, B. & Kinzler, K. W. The multistep nature of cancer. Trends Genet. 9, 138–141 (1993).
Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J. & Schreiber, R. D. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunol. 3, 991–998 (2002). This is an insightful review on the mechanisms of immune surveillance and immune editing of tumours.
Pardoll, D. Does the immune system see tumors as foreign or self? Annu. Rev. Immunol. 21, 807–839 (2003).
Blattman, J. N. & Greenberg, P. D. Cancer immunotherapy: a treatment for the masses. Science 305, 200–205 (2004).
Gabrilovich, D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nature Rev. Immunol. 4, 941–952 (2004).
Zou, W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nature Rev. Cancer 5, 263–274 (2005).
Bissell, M. J. & Radisky, D. Putting tumours in context. Nature Rev. Cancer 1, 46–54 (2001).
Pollard, J. W. Tumour-educated macrophages promote tumour progression and metastasis. Nature Rev. Cancer 4, 71–78 (2004).
Yu, C. L. et al. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science 269, 81–83 (1995). This study provides the first evidence that STAT3 has a role in oncogenesis in that STAT3 is constitutively activated by SRC oncoprotein.
Bromberg, J. & Darnell, J. E. The role of STATs in transcriptional control and their impact on cellular function. Oncogene 19, 2468–2473 (2000).
Yu, H. & Jove, R. The STATs of cancer — new molecular targets come of age. Nature Rev. Cancer 4, 97–105 (2004). A comprehensive review on the role of STAT3 in cancer progression and on STAT3 as a target for cancer therapy.
Takeda, K. et al. Enhanced TH1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10, 39–49 (1999).
Welte, T. et al. STAT3 deletion during hematopoiesis causes Crohn's disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc. Natl Acad. Sci. USA 100, 1879–1884 (2003).
Wang, T. et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nature Med. 10, 48–54 (2004). This study provided the first evidence at the molecular level that oncogenesis, for which STAT3 is crucial, coordinates tumour immune evasion. STAT3 activity in tumour cells not only inhibits the expression of T H 1-type immune-stimulating molecules, it also promotes the expression of immunosuppressive factors, leading to the inhibition of DC maturation.
Kortylewski, M. et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nature Med. 11, 1314–1321 (2005). This study shows that STAT3 is constitutively activated in diverse immune-cell subsets in the tumour microenvironment. STAT3 signalling allows tumour cells and local immune cells to resonate with one another, leading to immunosuppression.
Nabarro, S. et al. Coordinated oncogenic transformation and inhibition of host immune responses by the PAX3–FKHR fusion oncoprotein. J. Exp. Med. 202, 1399–1410 (2005). This paper shows that activation of STAT3 by an oncoprotein inhibits the host immune response in mouse models.
Sumimoto, H., Imabayashi, F., Iwata, T. & Kawakami, Y. The BRAF–MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J. Exp. Med. 203, 1651–1656 (2006). Results from this paper show that both STAT3 activation and the BRAF–MAPK signalling pathway can promote the expression of immunosuppressive factors such as IL-6, IL-10 and VEGF in human melanoma cells. Blocking either of these oncogenic signalling pathways in DCs decreases tumour-factor-induced inhibition of IL-12 expression.
Darnell, J. E., Kerr, I. M. & Stark, G. R. Jak–STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264, 1415–1421 (1994).
Taga, T. & Kishimoto, T. Gp130 and the interleukin-6 family of cytokines. Annu. Rev. Immunol. 15, 797–819 (1997).
Heinrich, P. C., Behrmann, I., Muller-Newen, G., Schaper, F. & Graeve, L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 334, 297–314 (1998).
Darnell, J. E. Studies of IFN-induced transcriptional activation uncover the Jak–Stat pathway. J. Interferon Cytokine Res. 18, 549–554 (1998).
Stark, G. R., Kerr, I. M., Williams, B. R., Silverman, R. H. & Schreiber, R. D. How cells respond to interferons. Annu. Rev. Biochem. 67, 227–264 (1998).
Heinrich, P. C. et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374, 1–20 (2003).
Wang, R., Cherukuri, P. & Luo, J. Activation of Stat3 sequence-specific DNA binding and transcription by p300/CREB-binding protein-mediated acetylation. J. Biol. Chem. 280, 11528–11534 (2005).
Yuan, Z. L., Guan, Y. J., Chatterjee, D. & Chin, Y. E. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science 307, 269–273 (2005).
Silvennoinen, O., Schindler, C., Schlessinger, J. & Levy, D. E. Ras-independent growth factor signaling by transcription factor tyrosine phosphorylation. Science 261, 1736–1739 (1993).
Zhong, Z., Wen, Z. & Darnell, J. E. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264, 95–98 (1994).
Ruff-Jamison, S. et al. Epidermal growth factor and lipopolysaccharide activate Stat3 transcription factor in mouse liver. J. Biol. Chem. 269, 21933–21935 (1994).
Alexander, W. S. Suppressors of cytokine signalling (SOCS) in the immune system. Nature Rev. Immunol. 2, 410–416 (2002).
Kubo, M., Hanada, T. & Yoshimura, A. Suppressors of cytokine signaling and immunity. Nature Immunol. 4, 1169–1176 (2003).
Shuai, K. & Liu, B. Regulation of gene-activation pathways by PIAS proteins in the immune system. Nature Rev. Immunol. 5, 593–605 (2005).
Turkson, J. et al. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol. Cell. Biol. 18, 2545–2552 (1998).
Bromberg, J. F., Horvath, C. M., Besser, D., Lathem, W. W. & Darnell, J. E. Stat3 activation is required for cellular transformation by v-src. Mol. Cell. Biol. 18, 2553–2558 (1998).
Bromberg, J. F. et al. Stat3 as an oncogene. Cell 98, 295–303 (1999). Using a constitutively active mutant form of STAT3, this study formally establishes that STAT3 is an oncoprotein.
Catlett-Falcone, R. et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 10, 105–115 (1999).
Grandis, J. R. et al. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor-mediated cell growth in vitro. J. Clin. Invest. 102, 1385–1392 (1998).
Coffer, P. J., Koenderman, L. & de Groot, R. P. The role of STATs in myeloid differentiation and leukemia. Oncogene 19, 2511–2522 (2000).
Lin, T. S., Mahajan, S. & Frank, D. A. STAT signaling in the pathogenesis and treatment of leukemias. Oncogene 19, 2496–2504 (2000).
Kortylewski, M., Jove, R. & Yu, H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 24, 315–327 (2005).
Niu, G. et al. Gene therapy with dominant-negative Stat3 suppresses growth of the murine melanoma B16 tumor in vivo. Cancer Res. 59, 5059–5063 (1999).
Niu, G. et al. Overexpression of a dominant-negative signal transducer and activator of transcription 3 variant in tumor cells leads to production of soluble factors that induce apoptosis and cell cycle arrest. Cancer Res. 61, 3276–3280 (2001).
Niu, G. et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 21, 2000–2008 (2002).
Wei, D. et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene 22, 319–329 (2003).
Wei, L. H. et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene 22, 1517–1527 (2003).
Xu, Q. et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene 24, 5552–5560 (2005).
Wojcik, E. J. et al. A novel activating function of c-Src and Stat3 on HGF transcription in mammary carcinoma cells. Oncogene 25, 2773–2784 (2006).
Xie, T. X. et al. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene 23, 3550–3560 (2004).
Dechow, T. N. et al. Requirement of matrix metalloproteinase-9 for the transformation of human mammary epithelial cells by Stat3-C. Proc. Natl Acad. Sci. USA 101, 10602–10607 (2004).
Burdelya, L. et al. Stat3 activity in melanoma cells affects migration of immune effector cells and nitric oxide-mediated antitumor effects. J. Immunol. 174, 3925–3931 (2005).
Vicari, A. P., Caux, C. & Trinchieri, G. Tumour escape from immune surveillance through dendritic cell inactivation. Semin. Cancer Biol. 12, 33–42 (2002).
Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Nefedova, Y. et al. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J. Immunol. 172, 464–474 (2004).
Park, S. J. et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J. Immunol. 173, 3844–3854 (2004).
Kawakami, Y. et al. Regulation of dendritic cell maturation and function by Bruton's tyrosine kinase via IL-10 and Stat3. Proc. Natl Acad. Sci. USA 103, 153–158 (2006).
Cheng, F. et al. A critical role for Stat3 signaling in immune tolerance. Immunity 19, 425–436 (2003).
Sun, Z., Yao, Z., Liu, S., Tang, H. & Yan, X. An oligonucleotide decoy for Stat3 activates the immune response of macrophages to breast cancer. Immunobiology 211, 199–209 (2006).
Yu, P. et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J. Exp. Med. 201, 779–791 (2005).
Nishikawa, H. et al. IFN-γ controls the generation/activation of CD4+ CD25+ regulatory T cells in antitumor immune response. J. Immunol. 175, 4433–4440 (2005).
Larmonier, N. et al. Tumor-derived CD4+CD25+ regulatory T cell suppression of dendritic cell function involves TGF-β and IL-10. Cancer Immunol. Immunother. 56, 48–59 (2007).
Liyanage, U. K. et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J. Immunol. 169, 2756–2761 (2002).
Woo, E. Y. et al. Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 61, 4766–4772 (2001).
Zou, W. Regulatory T cells, tumour immunity and immunotherapy. Nature Rev. Immunol. 6, 295–307 (2006).
Curiel, T. J. et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Med. 10, 942–949 (2004).
Wei, S. et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 65, 5020–5026 (2005).
Sakaguchi, S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nature Immunol. 6, 345–352 (2005).
Shevach, E. M. CD4+ CD25+ suppressor T cells: more questions than answers. Nature Rev. Immunol. 2, 389–400 (2002).
Chen, W. et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 (2003).
Fantini, M. C. et al. Cutting edge: TGF-β induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 172, 5149–5153 (2004).
Kinjyo, I. et al. Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-β1. J. Exp. Med. 203, 1021–1031 (2006). This study shows that increased STAT3 signalling, as a result of Socs3 ablation, is crucial for the expression of IL-10 and TGF-β by T H cells, indicating a crucial role for STAT3 in T Reg cells.
Kasprzycka, M., Marzec, M., Liu, X., Zhang, Q. & Wasik, M. A. Nucleophosmin/anaplastic lymphoma kinase (NPM/ALK) oncoprotein induces the T regulatory cell phenotype by activating STAT3. Proc. Natl Acad. Sci. USA 103, 9964–9969 (2006).
Doganci, A. et al. The IL-6Rα chain controls lung CD4+CD25+ TR eg development and function during allergic airway inflammation in vivo. J. Clin. Invest. 115, 313–325 (2005).
Zorn, E. et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood 108, 1571–1579 (2006). This paper shows that IL-2-induced FOXP3 expression in human T Reg cells is mediated by STAT3 and STAT5.
Antov, A., Yang, L., Vig, M., Baltimore, D. & Van Parijs, L. Essential role for STAT5 signaling in CD25+CD4+ regulatory T cell homeostasis and the maintenance of self-tolerance. J. Immunol. 171, 3435–3441 (2003).
Snow, J. W. et al. Loss of tolerance and autoimmunity affecting multiple organs in STAT5A/5B-deficient mice. J. Immunol. 171, 5042–5050 (2003).
Chan, S. M., Ermann, J., Su, L., Fathman, C. G. & Utz, P. J. Protein microarrays for multiplex analysis of signal transduction pathways. Nature Med. 10, 1390–1396 (2004).
Anderson, P. O. et al. IL-2 overcomes the unresponsiveness but fails to reverse the regulatory function of antigen-induced T regulatory cells. J. Immunol. 174, 310–319 (2005).
Dercamp, C., Chemin, K., Caux, C., Trinchieri, G. & Vicari, A. P. Distinct and overlapping roles of interleukin-10 and CD25+ regulatory T cells in the inhibition of antitumor CD8 T-cell responses. Cancer Res. 65, 8479–8486 (2005).
Langowski, J. L. et al. IL-23 promotes tumour incidence and growth. Nature 442, 461–465 (2006).
Hoentjen, F., Sartor, R. B., Ozaki, M. & Jobin, C. STAT3 regulates NF-κB recruitment to the IL-12p40 promoter in dendritic cells. Blood 105, 689–696 (2005).
Chen, Z. et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc. Natl Acad. Sci. USA 103, 8137–8142 (2006).
Karin, M. & Greten, F. R. NF-κB: linking inflammation and immunity to cancer development and progression. Nature Rev. Immunol. 5, 749–759 (2005).
Dalwadi, H. et al. Cyclooxygenase-2-dependent activation of signal transducer and activator of transcription 3 by interleukin-6 in non-small cell lung cancer. Clin. Cancer Res. 11, 7674–7682 (2005).
Shankaran, V. et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410, 1107–1111 (2001).
Alonzi, T. et al. Induced somatic inactivation of STAT3 in mice triggers the development of a fulminant form of enterocolitis. Cytokine 26, 45–56 (2004).
Turkson, J. et al. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. J. Biol. Chem. 276, 45443–45455 (2001).
Turkson, J. et al. A novel platinum compound inhibits constitutive Stat3 signaling and induces cell cycle arrest and apoptosis of malignant cells. J. Biol. Chem. 280, 32979–32988 (2005).
Turkson, J. et al. Inhibition of constitutive signal transducer and activator of transcription 3 activation by novel platinum complexes with potent antitumor activity. Mol. Cancer Ther. 3, 1533–1542 (2004).
van Elsas, A., Hurwitz, A. A. & Allison, J. P. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 190, 355–366 (1999).
Riley, J. L. & June, C. H. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood 105, 13–21 (2005).
Shen, L., Evel-Kabler, K., Strube, R. & Chen, S. Y. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nature Biotechnol. 22, 1546–1553 (2004).
Kryczek, I. et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J. Exp. Med. 203, 871–881 (2006).
Mellor, A. L. & Munn, D. H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nature Rev. Immunol. 4, 762–774 (2004).
Rosenberg, S. A., Yang, J. C. & Restifo, N. P. Cancer immunotherapy: moving beyond current vaccines. Nature Med. 10, 909–915 (2004).
Pardoll, D. M. Spinning molecular immunology into successful immunotherapy. Nature Rev. Immunol. 2, 227–238 (2002).
Steinman, R. M. & Mellman, I. Immunotherapy: bewitched, bothered, and bewildered no more. Science 305, 197–200 (2004).
Vicari, A. P. et al. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J. Exp. Med. 196, 541–549 (2002).
Nefedova, Y. et al. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 65, 9525–9535 (2005).
Kitamura, H. et al. IL-6–STAT3 controls intracellular MHC class II αβ dimer level through cathepsin S activity in dendritic cells. Immunity 23, 491–502 (2005).
Kammertoens, T., Schuler, T. & Blankenstein, T. Immunotherapy: target the stroma to hit the tumor. Trends Mol. Med. 11, 225–231 (2005).
Wang, S. et al. Tumor evasion of the immune system: inhibiting p38 MAPK signaling restores the function of dendritic cells in multiple myeloma. Blood 107, 2432–2439 (2006).
Dauer, D. J. et al. Stat3 regulates genes common to both wound healing and cancer. Oncogene 24, 3397–3408 (2005).
Sano, S. et al. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J. 18, 4657–4668 (1999).
Chiarle, R. et al. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nature Med. 11, 623–629 (2005).
Okada, S. et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nature Med. 12, 829–834 (2006).
Odajima, J. et al. Full oncogenic activities of v-Src are mediated by multiple signaling pathways. Ras as an essential mediator for cell survival. J. Biol. Chem. 275, 24096–24105 (2000).
Ning, Z. Q., Li, J., McGuinness, M. & Arceci, R. J. STAT3 activation is required for Asp816 mutant c-Kit induced tumorigenicity. Oncogene 20, 4528–4536 (2001).
Bowman, T. et al. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc. Natl Acad. Sci. USA 98, 7319–7324 (2001).
Sinibaldi, D. et al. Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene 19, 5419–5427 (2000).
Karni, R., Jove, R. & Levitzki, A. Inhibition of pp60c-Src reduces Bcl-XL expression and reverses the transformed phenotype of cells overexpressing EGF and HER-2 receptors. Oncogene 18, 4654–4662 (1999).
Epling-Burnette, P. K. et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J. Clin. Invest. 107, 351–362 (2001).
Niu, G. et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene 21, 7001–7010 (2002).
Aoki, Y., Feldman, G. M. & Tosato, G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood 101, 1535–1542 (2003).
Amin, H. M. et al. Selective inhibition of STAT3 induces apoptosis and G1 cell cycle arrest in ALK-positive anaplastic large cell lymphoma. Oncogene 23, 5426–5434 (2004).
Gritsko, T. et al. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin. Cancer Res. 12, 11–19 (2006).
Niu, G. et al. Role of Stat3 in regulating p53 expression and function. Mol. Cell. Biol. 25, 7432–7440 (2005).
Hung, W. & Elliott, B. Co-operative effect of c-Src tyrosine kinase and Stat3 in activation of hepatocyte growth factor expression in mammary carcinoma cells. J. Biol. Chem. 276, 12395–12403 (2001).
Xie, T. X. et al. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 66, 3188–3196 (2006).
Jung, J. E. et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 19, 1296–1298 (2005).
Herbeuval, J. P., Lelievre, E., Lambert, C., Dy, M. & Genin, C. Recruitment of STAT3 for production of IL-10 by colon carcinoma cells induced by macrophage-derived IL-6. J. Immunol. 172, 4630–4636 (2004).
Acknowledgements
We would like to thank members of our laboratory, especially G.-L. Niu, M. Kujawski, T.-H. Wang and L. Burdelya, for their contributions to the work summarized here. We would also like to acknowledge the pioneering work of R. Jove in linking STAT3 with cancer that inspired the initial gene-therapy experiment. H.Y. was supported by US National Institutes of Health (NIH) grants and by the Dr Tsai-fan Yu Cancer Research Endowment. D.P. was supported by NIH grants and gifts from the Topercer family, D. Needle, J. Goldsmith, the Seraph Foundation and the Janney Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Danger signals
-
A danger signal is normally defined as the pathogen-associated molecular pattern that is recognized by host receptors. Danger signals often trigger the production of cytokines, chemokines and other physiological mediators, such as nitric oxide, leading to immune responses against the pathogen. In the context of this Review, 'danger signals' refer to the similar cytokines, chemokines and other T helper 1-type immunostimulating molecules that are produced by transformed cells on STAT3 inhibition.
- Tolerogenic dendritic cells
-
Dendritic cells that can attenuate T-cell-mediated immune responses by anergizing or changing the effector function of antigen-specific T cells.
- Mx1–Cre–loxP system
-
The Mx1–Cre–loxP system allows specific gene ablation, mostly in the haematopoietic cell lineages of adult mice. Injection of polyinosinic–polycytidylic-acid oligonucleotides stimulates the production of type-I interferons, which induce Cre recombinase expression through the interferon-sensitive Mx1 promoter, resulting in the ablation of target gene alleles flanked by loxP sites.
- T-cell anergy
-
A state of T-cell unresponsiveness to stimulation with antigen. It can be induced by stimulation with a large amount of specific antigen in the absence of the engagement of co-stimulatory molecules.
- Regulatory T (TReg) cells
-
A rare population of CD4+ T cells that naturally express high levels of CD25 (the interleukin-2 receptor α-chain) and the transcription factor forkhead box P3 (FOXP3), and that have suppressive regulatory activity towards effector T cells and other immune cells. Absence or dysfunction of TReg cells is associated with severe autoimmunity. In tumours, TReg cells are induced and proliferate, thereby suppressing anti-tumour immunity.
- Plasmacytoid dendritic cells
-
A subset of dendritic cells (DCs) that are described as plasmacytoid because of their microscopic appearance that resembles plasmablasts. In humans, these DCs can be derived from lineage-negative stem cells in peripheral blood and are the main producers of type-I interferon (IFN) in response to virus infections. Recent studies have identified a subset of type-I IFN-producing DCs in mice, which are characterized by expression of B220 and Ly6C.
- Nucleophosmin/anaplastic lymphoma kinase (NPM/ALK) oncoprotein
-
An oncogenic fusion tyrosine kinase that is associated with a specific type of non-Hodgkin's lymphoma. The translocation between chromosomes 5 and 2 results in fusion of the amino-terminal part of the ubiquitous nucleolar protein NPM to the cytoplasmic fragment of the receptor tyrosine kinase ALK, creating a hybrid tyrosine kinase with constitutive activity.
- Immune-mediated colitis
-
An inflammatory disease of the colon most commonly classified as ulcerative colitis or Crohn's disease. Various hereditary and induced mouse models of human colitis have been developed.
- RNA interference
-
(RNAi). Double-stranded RNAs (dsRNAs) with sequences that precisely match a given gene are able to 'knock down' the expression of that gene by directing RNA-degrading enzymes to destroy the encoded mRNA transcript. The two most common forms of dsRNAs used for gene silencing are short — usually 21-bp long — small interfering RNAs (siRNAs) or the plasmid-delivered short hairpin RNAs (shRNAs).
Rights and permissions
About this article
Cite this article
Yu, H., Kortylewski, M. & Pardoll, D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 7, 41–51 (2007). https://doi.org/10.1038/nri1995
Issue Date:
DOI: https://doi.org/10.1038/nri1995
This article is cited by
-
Unraveling the complexity of STAT3 in cancer: molecular understanding and drug discovery
Journal of Experimental & Clinical Cancer Research (2024)
-
JAK/STAT3 represents a therapeutic target for colorectal cancer patients with stromal-rich tumors
Journal of Experimental & Clinical Cancer Research (2024)
-
Evolving cognition of the JAK-STAT signaling pathway: autoimmune disorders and cancer
Signal Transduction and Targeted Therapy (2023)
-
Hyaluronic acid-bilirubin nanomedicine-based combination chemoimmunotherapy
Nature Communications (2023)
-
JMJD6 in tumor-associated macrophage regulates macrophage polarization and cancer progression via STAT3/IL-10 axis
Oncogene (2023)