Key Points
-
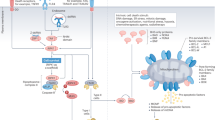
As cells are infected by microorganisms, they can pre-emptively die to prevent the replication and spreading of the pathogen. Cell death that is associated with the presence of pathogen-associated molecular patterns (PAMPs) can stimulate vigorous immune responses.
-
In the absence of PAMPs, damage-associated molecular patterns (DAMPs) produced by dying cells can stimulate an immune response that can elicit the specific recognition of antigens expressed by dying cells (for example, tumour antigens).
-
The nature of the immune response to cell death depends on which cells die, where they die, how they die, which cell engulfs them and when (or if) an associated antigen has been or will be recognized. Variations in these factors can have consequences that range from effective anti-pathogen or antitumour responses to autoimmune pathology.
-
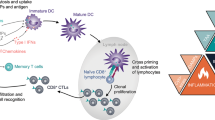
The simple idea that apoptosis is tolerogenic or non-immunogenic and that necrosis is immunogenic is an oversimplification. Thus, apoptosis of tumour cells that is induced by chemotherapy can prime an efficient immune response, which in turn can contribute to the efficacy of antitumour regimens.
-
Various DAMPs contribute to the immunogenicity of apoptotic cell death. These include the surface exposure of chaperone proteins (such as calreticulin) or the release of proteins, such as high-mobility group box 1 protein (HMGB1) and SIN3A-associated protein 130 (SAP130) among others. The catabolic action of caspases and autophagy can also contribute to the immunogenicity of cell death.
-
The tolerogenic effect of cell death depends on many factors, including the absence of T cell help, the location of the dying cells (which in part dictates their engulfment by distinct dendritic cell (DC) subtypes), the maturation state of the DC, the production of immunosuppressive factors (such as transforming growth factor-β) or the modification of DAMPs (for example, oxidation of HMGB1 that results in its inactivation).
-
In conclusion, the mechanisms that determine the immune response to dead and dying cells are complex. Understanding (and possibly manipulating) these mechanisms can have important implications for cancer biology, infectious disease, tissue injury and autoimmunity.
Abstract
The immune system is routinely exposed to dead cells during normal cell turnover, injury and infection. Mechanisms must exist to discriminate between different forms of cell death to correctly eliminate pathogens and promote healing while avoiding responses to self, which can result in autoimmunity. However, an effective immune response against host tissue is often needed to eliminate tumours following treatment with chemotherapeutic agents that trigger tumour cell death. Consequently, a central problem in immunology is to understand how the immune system determines whether cell death is immunogenic, tolerogenic or 'silent'.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Reed, J. C. Drug insight: cancer therapy strategies based on restoration of endogenous cell death mechanisms. Nature Clin. Pract. Oncol. 3, 388–398 (2006).
Medzhitov, R. & Janeway, C. A. Jr. Decoding the patterns of self and nonself by the innate immune system. Science 296, 298–300 (2002).
Zitvogel, L. et al. Immune response against dying tumor cells. Adv. Immunol. 84, 131–179 (2004).
Gaipl, U. S. et al. Clearance deficiency and systemic lupus erythematosus (SLE). J. Autoimmun. 28, 114–121 (2007).
Matzinger, P. The danger model: a renewed sense of self. Science 296, 301–305 (2002).
Thompson, C. B. Apoptosis in the pathogenesis and treatment of disease. Science 267, 1456–1462 (1995).
Casares, N. et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 202, 1691–1701 (2005).
Obeid, M. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nature Med. 13, 54–61 (2007). This paper contains information suggesting that subtle differences in the surface characteristics of dying cells can determine how they are handled by DCs and whether an adaptive immune response will ensue.
Kerr, J. F., Wyllie, A. H. & Currie, A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239–257 (1972).
Griffith, T. S., Yu, X., Herndon, J. M., Green, D. R. & Ferguson, T. A. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity 5, 7–16 (1996). This is an early paper showing that apoptotic cells induce tolerance to associated antigens.
Ferguson, T. A. et al. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8+ T cells produce active immune unresponsiveness. J. Immunol. 168, 5589–5595 (2002).
Belz, G. T. et al. The CD8α+ dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J. Exp. Med. 196, 1099–1104 (2002). References 11 and 12 show that CD8α+ DCs cross-present antigen on MHC class I molecules to induce tolerance.
Garza, K. M. et al. Role of antigen-presenting cells in mediating tolerance and autoimmunity. J. Exp. Med. 191, 2021–2027 (2000).
Miyake, Y. et al. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J. Clin. Invest. 117, 2268–2278 (2007).
Albert, M. L., Jegathesan, M. & Darnell, R. B. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nature Immunol. 2, 1010–1017 (2001).
Heath, W. R., Kurts, C., Miller, J. F. & Carbone, F. R. Cross-tolerance: a pathway for inducing tolerance to peripheral tissue antigens. J. Exp. Med. 187, 1549–1553 (1998).
Steinman, R. M., Turley, S., Mellman, I. & Inaba, K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med. 191, 411–416 (2000). This is an important early review on tolerance induction that is mediated by the uptake of apoptotic cells.
Battisto, J. R. & Bloom, B. R. Dual immunological unresponsiveness induced by cell membrane coupled hapten or antigen. Nature 212, 156–157 (1966). Pre-dating most relevant studies by decades, this work shows that injected cells can cause immune tolerance.
Conlon, P. J., Miller, S. D. & Claman, H. N. The induction of tolerance to DNFB contact sensitivity by using hapten-modified lymphoid cells. III. Effects of hapten concentration on the ability of MLS-disparate cells to induce rapid unresponsiveness. J. Immunol. 125, 807–813 (1980).
Pincott, C. E. & Bainbridge, D. R. Studies on transplantation immunity. IV. Murine natural immunity to lymphoid cells in vivo. Eur. J. Immunol. 10, 250–257 (1980).
Sun, E. et al. Allograft tolerance induced by donor apoptotic lymphocytes requires phagocytosis in the recipient. Cell Death Differ. 11, 1258–1264 (2004).
van Twuyver, E. et al. Pretransplantation blood transfusion revisited. N. Engl. J. Med. 325, 1210–1213 (1991).
Pradhan, S. et al. A critical role for the proapoptotic protein Bid in ultraviolet-induced immune suppression and cutaneous apoptosis. J. Immunol. 181, 3077–3088 (2008).
Schwarz, A. et al. Ultraviolet light-induced immune tolerance is mediated via the Fas/Fas-ligand system. J. Immunol. 160, 4262–4270 (1998).
Heath, W. R. & Carbone, F. R. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 19, 47–64 (2001).
Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Segal, N. H. et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 68, 889–892 (2008).
Apetoh, L. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature Med. 13, 1050–1059 (2007). This paper provides evidence that TLR4 expression by DCs dictates whether the immune system can mount a CTL response against antigens from dying tumour cells and suggests that one of the ligands for TLR4 released by dying cells is HMGB1.
Zitvogel, L., Apetoh, L., Ghiringhelli, F. & Kroemer, G. Immunological aspects of cancer chemotherapy. Nature Rev. Immunol. 8, 59–73 (2008).
Johansson, U., Walther-Jallow, L., Smed-Sorensen, A. & Spetz, A. L. Triggering of dendritic cell responses after exposure to activated, but not resting, apoptotic PBMCs. J. Immunol. 179, 1711–1720 (2007).
Herndon, J. M., Stuart, P. M. & Ferguson, T. A. Peripheral deletion of antigen-specific T cells leads to long-term tolerance mediated by CD8+ cytotoxic cells. J. Immunol. 174, 4098–4104 (2005).
Gasser, S., Orsulic, S., Brown, E. J. & Raulet, D. H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436, 1186–1190 (2005).
Wajapeyee, N., Serra, R. W., Zhu, X., Mahalingam, M. & Green, M. R. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 132, 363–374 (2008).
Kuilman, T. et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031 (2008).
Acosta, J. C. et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133, 1006–1018 (2008).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007). An interesting study in which induction of cellular senescence triggers immune-mediated tumour cell clearance.
Srivastava, P. K. Therapeutic cancer vaccines. Curr. Opin. Immunol. 18, 201–205 (2006).
Obeid, M. et al. Ecto-calreticulin in immunogenic chemotherapy. Immunol. Rev. 220, 22–34 (2007).
Spisek, R. et al. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood 109, 4839–4845 (2007).
Panaretakis, T. et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ. 15, 1499–1509 (2008).
Lauber, K. et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 113, 717–730 (2003).
Le, L. Q. et al. Mice lacking the orphan G protein-coupled receptor G2A develop a late-onset autoimmune syndrome. Immunity 14, 561–571 (2001).
Peter, C. et al. Migration to apoptotic “find-me” signals is mediated via the phagocyte receptor G2A. J. Biol. Chem. 283, 5296–5305 (2008).
Koizumi, S. et al. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 446, 1091–1095 (2007).
Hanley, P. J. et al. Extracellular ATP induces oscillations of intracellular Ca2+ and membrane potential and promotes transcription of IL-6 in macrophages. Proc. Natl Acad. Sci. USA 101, 9479–9484 (2004).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Martinon, F., Gaide, O., Petrilli, V., Mayor, A. & Tschopp, J. NALP inflammasomes: a central role in innate immunity. Semin. Immunopathol. 29, 213–229 (2007).
Scaffidi, P., Misteli, T. & Bianchi, M. E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418, 191–195 (2002). This is a key study that identifies HMGB1 as a DAMP.
Bell, C. W., Jiang, W., Reich, C. F. 3rd & Pisetsky, D. S. The extracellular release of HMGB1 during apoptotic cell death. Am. J. Physiol. Cell Physiol. 291, C1318–C1325 (2006).
Jiang, W., Bell, C. W. & Pisetsky, D. S. The relationship between apoptosis and high-mobility group protein 1 release from murine macrophages stimulated with lipopolysaccharide or polyinosinic–polycytidylic acid. J. Immunol. 178, 6495–6503 (2007).
Kazama, H. et al. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity 29, 21–32 (2008). This study 'connects the dots' from caspase activation to ROS production to modification of HMGB1 and to immune tolerance.
Bianchi, M. E. & Manfredi, A. A. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol. Rev. 220, 35–46 (2007).
Marshak-Rothstein, A. Toll-like receptors in systemic autoimmune disease. Nature Rev. Immunol. 6, 823–835 (2006).
Tian, J. et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nature Immunol. 8, 487–496 (2007).
Yamasaki, S. et al. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nature Immunol. 9, 1179–1188 (2008).
Sancho, D. et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature 15 Feb 2009 (doi:10.1038/nature07750). This paper reports the identification of a new PRR on DCs that senses the presence of an unknown intracellular ligand that becomes accessible in dead cells following permeabilization of the plasma membrane and directs antigens for cross-presentation.
Kroemer, G. et al. Classifications of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Diff. 16, 3–11 (2008).
Castiglioni, P. et al. Apoptosis-dependent subversion of the T-lymphocyte epitope hierarchy in lymphoma cells. Cancer Res. 62, 1116–1122 (2002).
Rawson, P. M. et al. Cross-presentation of caspase-cleaved apoptotic self antigens in HIV infection. Nature Med. 13, 1431–1439 (2007).
Tenev, T., Ditzel, M., Zachariou, A. & Meier, P. The antiapoptotic activity of insect IAPs requires activation by an evolutionarily conserved mechanism. Cell Death Differ. 14, 1191–1201 (2007).
Obeid, M. et al. Calreticulin exposure is required for the immunogenicity of γ-irradiation and UVC light-induced apoptosis. Cell Death Differ. 14, 1848–1850 (2007).
Kroemer, G. & Levine, B. Autophagic cell death: the story of a misnomer. Nature Rev. Mol. Cell Biol. 9, 1004–1010 (2008).
Jin, S., DiPaola, R. S., Mathew, R. & White, E. Metabolic catastrophe as a means to cancer cell death. J. Cell Sci. 120, 379–383 (2007).
Qu, X. et al. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell 128, 931–946 (2007).
Thorburn, J. et al. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ. 16, 175–183 (2008).
Shimizu, S. et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nature Cell Biol. 6, 1221–1228 (2004).
Uhl, M., Kepp, O., Jusforgues-Saklani, H., Kroemer, G. & Albert, M. L. Autophagic death facilitates efficient antigen cross-priming by stimulating type I IFN production by phagocytic dendritic cells. Cell Death Diff. 20 Feb 2009 (doi:10.1038/cdd.2009.8).
Albert, M. L. et al. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 188, 1359–1368 (1998).
Schoenberger, S. P., Toes, R. E., van der Voort, E. I., Offringa, R. & Melief, C. J. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature 393, 480–483 (1998).
Sun, J. C. & Bevan, M. J. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300, 339–342 (2003).
Janssen, E. M. et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 434, 88–93 (2005). This studied characterizes the production of TRAIL following restimulation of helpless CTLs and defines its role both in killing the CTLs and inhibiting T cell responses.
Janssen, E. M. et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421, 852–856 (2003).
Griffith, T. S. et al. Apoptotic cells induce tolerance by generating helpless CD8+ T cells that produce TRAIL. J. Immunol. 178, 2679–2687 (2007). This study shows that of the induction of tolerance by apoptotic cells involves helpless CTLs and the production of TRAIL.
Greene, M. I. & Benacerraf, B. Studies on hapten specific T cell immunity and suppression. Immunol. Rev. 50, 163–186 (1980).
Scott, C. F. Jr, Tsurufuji, M., Benacerraf, B. & Sy, M. S. Regulation of the hapten-specific T cell response. I. Preferential induction of hyporesponsiveness to the D-end of the major histocompatibility complex in the hapten-specific cytotoxic T cell response. J. Immunol. 131, 2184–2189 (1983).
Chaput, N. et al. Molecular determinants of immunogenic cell death: surface exposure of calreticulin makes the difference. J. Mol. Med. 85, 1069–1076 (2007).
Apetoh, L. et al. Immunogenic chemotherapy: discovery of a critical protein through proteomic analyses of tumor cells. Cancer Genomics Proteomics 4, 65–70 (2007).
Ferguson, T. A., Hayashi, J. D. & Kaplan, H. J. The immune response and the eye. III. Anterior chamber-associated immune deviation can be adoptively transferred by serum. J. Immunol. 143, 821–826 (1989).
den Haan, J. M., Lehar, S. M. & Bevan, M. J. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192, 1685–1696 (2000).
Dudziak, D. et al. Differential antigen processing by dendritic cell subsets in vivo. Science 315, 107–111 (2007).
Iyoda, T. et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J. Exp. Med. 195, 1289–1302 (2002).
Allan, R. S. et al. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science 301, 1925–1928 (2003).
Huang, L., Soldevila, G., Leeker, M., Flavell, R. & Crispe, I. N. The liver eliminates T cells undergoing antigen-triggered apoptosis in vivo. Immunity 1, 741–749 (1994).
Crispe, I. N. et al. Cellular and molecular mechanisms of liver tolerance. Immunol. Rev. 213, 101–118 (2006).
Sauter, B. et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191, 423–434 (2000).
Yamaguchi, H. et al. Milk fat globule EGF factor 8 in the serum of human patients of systemic lupus erythematosus. J. Leukoc. Biol. 83, 1300–1307 (2008).
Gaipl, U. S. et al. Inefficient clearance of dying cells and autoreactivity. Curr. Top. Microbiol. Immunol. 305, 161–176 (2006).
Hanayama, R. et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 304, 1147–1150 (2004). This paper outlines the functional link between defective clearance of apoptotic cells and systemic autoimmune disease.
Miyanishi, M. et al. Identification of Tim4 as a phosphatidylserine receptor. Nature 450, 435–439 (2007).
Ravichandran, K. S. & Lorenz, U. Engulfment of apoptotic cells: signals for a good meal. Nature Rev. Immunol. 7, 964–974 (2007).
Lucas, M. et al. Requirements for apoptotic cell contact in regulation of macrophage responses. J. Immunol. 177, 4047–4054 (2006).
Ricci, J. E. et al. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell 117, 773–786 (2004).
Chung, E. Y. et al. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity 27, 952–964 (2007). This is a detailed study on the mechanistic link between phagocytosis of dying cells and the production of immunosuppressive cytokines by macrophages.
Fadok, V. A. et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 101, 890–898 (1998).
Voll, R. E. et al. Immunosuppressive effects of apoptotic cells. Nature 390, 350–351 (1997).
Serhan, C. N. & Savill, J. Resolution of inflammation: the beginning programs the end. Nature Immunol. 6, 1191–1197 (2005).
Huynh, M. L., Fadok, V. A. & Henson, P. M. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J. Clin. Invest. 109, 41–50 (2002).
Freire-de-Lima, C. G. et al. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature 403, 199–203 (2000).
Hoffmann, P. R. et al. Interaction between phosphatidylserine and the phosphatidylserine receptor inhibits immune responses in vivo. J. Immunol. 174, 1393–1404 (2005).
Gao, Y., Herndon, J. M., Zhang, H., Griffith, T. S. & Ferguson, T. A. Antiinflammatory effects of CD95 ligand (FasL)-induced apoptosis. J. Exp. Med. 188, 887–896 (1998).
Chen, W., Frank, M. E., Jin, W. & Wahl, S. M. TGF-β released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity 14, 715–725 (2001).
Kleinclauss, F. et al. Intravenous apoptotic spleen cell infusion induces a TGF-β-dependent regulatory T-cell expansion. Cell Death Differ. 13, 41–52 (2006).
Maeda, A. et al. Intravenous infusion of syngeneic apoptotic cells by photopheresis induces antigen-specific regulatory T cells. J. Immunol. 174, 5968–5976 (2005).
Torchinsky, M. B., Garaude, J., Martin, A. & Blander, J. M. Innate immune recognition of infected apoptotic cells directs TH17 cell differentiation. Nature 458, 78–82 (2009). This paper shows that engulfment of apoptotic cells by DCs induces T Reg or T helper 17 cells, depending on additional signals that are relevant to infection.
Hedrick, S. M. The acquired immune system: a vantage from beneath. Immunity 21, 607–615 (2004).
Shi, Y., Evans, J. E. & Rock, K. L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425, 516–521 (2003).
Jeannin, P., Jaillon, S. & Delneste, Y. Pattern recognition receptors in the immune response against dying cells. Curr. Opin. Immunol. 20, 530–537 (2008).
Ting, J. P., Willingham, S. B. & Bergstralh, D. T. NLRs at the intersection of cell death and immunity. Nature Rev. Immunol. 8, 372–379 (2008).
Di Virgilio, F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol. Sci. 28, 465–472 (2007).
Beutler, B. & Moresco, E. M. The forward genetic dissection of afferent innate immunity. Curr. Top. Microbiol. Immunol. 321, 3–26 (2008).
Galluzzi, L. et al. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 14, 1237–1243 (2007).
Festjens, N., Vanden Berghe, T. & Vandenabeele, P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim. Biophys. Acta 1757, 1371–1387 (2006).
Golstein, P. & Kroemer, G. Cell death by necrosis: towards a molecular definition. Trends Biochem. Sci. 32, 37–43 (2007).
Vakifahmetoglu, H., Olsson, M. & Zhivotovsky, B. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 15, 1153–1162 (2008).
Erwig, L. P. & Henson, P. M. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 15, 243–250 (2008).
Truman, L. A. et al. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood 112, 5026–5036 (2008).
Miksa, M., Amin, D., Wu, R., Ravikumar, T. S. & Wang, P. Fractalkine-induced MFG-E8 leads to enhanced apoptotic cell clearance by macrophages. Mol. Med. 13, 553–560 (2007).
Brown, S. et al. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature 418, 200–203 (2002).
Gardai, S. J. et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123, 321–334 (2005).
Martin, S. J. et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 182, 1545–1556 (1995).
Wolf, A., Schmitz, C. & Bottger, A. Changing story of the receptor for phosphatidylserine-dependent clearance of apoptotic cells. EMBO Rep. 8, 465–469 (2007).
Hanayama, R. et al. Identification of a factor that links apoptotic cells to phagocytes. Nature 417, 182–187 (2002).
Arur, S. et al. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev. Cell 4, 587–598 (2003).
Tibrewal, N. et al. Autophosphorylation docking site Tyr-867 in Mer receptor tyrosine kinase allows for dissociation of multiple signaling pathways for phagocytosis of apoptotic cells and down-modulation of lipopolysaccharide-inducible NF-κB transcriptional activation. J. Biol. Chem. 283, 3618–3627 (2008).
Acknowledgements
L.Z. and G.K. are supported by grants from the Ligue Nationale contre le Cancer, the European Union (ALLOSTEM, DC-THERA for L.Z.; Active p53, ApoSys, ApopTrain, RIGHT for G.K.), Fondation pour la Recherche Médicale, Cancéropôle Ile-de-France, Institut National du Cancer and Agence Nationale pour la Recherche. T.A.F. is supported by National Institutes of Health (NIH) grants EY06765, EY15570, and the Department of Ophthalmology and Visual Sciences core grant (EY02687). Support was also received from the Foundation for Fighting Blindness (Owings Mills, Maryland, USA), Research to Prevent Blindness (New York, USA), and the Macular Vision Research Foundation (West Conshohocken, Pennsylvania, USA). D.R.G. is supported by grants from the NIH and by the American Lebanese and Syrian Associated Charities.
Author information
Authors and Affiliations
Corresponding authors
Related links
Glossary
- Damage-associated molecular pattern
-
(DAMP). A molecule that is released from the degraded stroma (for example, hyaluronate), nucleus (for example, high-mobility group box 1 protein) and the cytoplasm (for example, ATP, uric acid, S100 calcium-binding proteins and heat shock proteins) as a result of cellular stress, cellular damage and non-physiological cell death. DAMPs are thought to elicit local inflammatory reactions.
- Dendritic cell
-
The main antigen-presenting cell of the immune system. Engulfment of dying cells by dendritic cells can lead to tolerance or immune responses to associated antigens.
- Delayed-type hypersensitivity
-
A cellular immune response to antigen that develops over 24 – 72 hours, is associated with the infiltration of T cells and monocytes and is dependent on the production of T helper 1-type cytokines.
- Tolerance
-
A term that denotes lymphocyte non-responsiveness to antigen, but implies an active process, not simply a passive lack of response.
- Hapten
-
A molecule that can bind antibody but cannot by itself elicit an immune response. Antibodies that are specific for a hapten can be generated when the hapten is chemically linked to a protein carrier that can elicit a T cell response.
- Contact hypersensitivity
-
A form of delayed-type hypersensitivity (type IV), in which T cells respond to antigens that are introduced through skin contact. This step requires dendritic cell mobilization from the skin to the draining lymph nodes to prime the antigen-specific T cells.
- CD95L
-
An apoptosis-inducing or death ligand of the tumour necrosis factor family.
- Caspase
-
An enzyme belonging to a family of cytoplasmic proteases that cleave their substrate after an aspartic acid residue. Initiator caspases are typically activated in response to particular stimuli. Effector caspases are activated by initiator caspases and are particularly important for the ordered dismantling of vital cellular structures.
- Cross-present
-
The presentation of exogenous antigen to CD8+ T cells by antigen-presenting cells (APCs). The antigen must be taken up by APCs and then re-routed to the MHC class I pathway of antigen presentation, resulting in the initiation of a CD8+ T cell response to an antigen that is not present within APCs.
- Activation-induced cell death
-
(AICD). The apoptotic cell death of activated lymphocytes. It ensures the rapid elimination of effector cells after their antigen-dependent clonal expansion. Defects in AICD result in lymphoproliferative diseases that are associated with autoimmune disorders.
- NKG2D
-
A lectin-type activating receptor that is expressed on the surface of natural killer (NK), NKT, γδ T cells and some cytolytic CD8+ αβ T cells. NKG2D recognizes the ligands MHC class I-polypeptide-related sequence A (MICA) and MICB in humans and retinoic acid early transcript 1 (RAE1) and H60 in mice. Such ligands are generally expressed by infected, stressed or transformed cells.
- Senescence
-
A nearly irreversible stage of permanent G1 cell cycle arrest, linked to morphological changes (flattening of the cells), metabolic changes and changes in gene expression, the induction of which depends on p53 and cell cycle blockers such as p21 and p16.
- p53
-
An important transcription factor that is activated by numerous genotoxic insults to induce cell cycle arrest, cell senescence or apoptosis. p53 is frequently mutated or functionally inactivated in cancer.
- Inflammasome
-
A molecular complex of several proteins that on assembly cleaves pro-interleukin-1 (IL-1), thereby producing active IL-1.
- HMGB1
-
A DAMP that is released from dying cells. It can be modified by reactive oxygen species to alter its immunostimulatory effects.
- Macroautophagy
-
The largely non-specific autophagic sequestration of cytoplasm into a double- or multiple-membrane-delimited compartment (an autophagosome) of non-lysosomal origin. Certain proteins, organelles and pathogens may be selectively degraded by macroautophagy.
- Immune-privileged site
-
An area in the body that has a decreased but not absent immune response to foreign antigens, including tissue grafts. These sites include the brain, eye, testis and placenta.
- 'Helpless' CTL
-
A CD8+ T cell that has undergone activation without additional stimulation ('help') by CD4+ T cells.
- Reactive oxygen species
-
Oxygen radicals that are produced by the mitochondrial respiratory chain and by other processes. When found in excessive quantities, they can cause intracellular and mitochondrial damage, which promotes cell death.
Rights and permissions
About this article
Cite this article
Green, D., Ferguson, T., Zitvogel, L. et al. Immunogenic and tolerogenic cell death. Nat Rev Immunol 9, 353–363 (2009). https://doi.org/10.1038/nri2545
Issue Date:
DOI: https://doi.org/10.1038/nri2545
This article is cited by
-
Nanomedicine targeting ferroptosis to overcome anticancer therapeutic resistance
Science China Life Sciences (2024)
-
Shikonin improves the effectiveness of PD-1 blockade in colorectal cancer by enhancing immunogenicity via Hsp70 upregulation
Molecular Biology Reports (2024)
-
Conventional type 1 dendritic cells (cDC1) in cancer immunity
Biology Direct (2023)
-
Analyzing molecular typing and clinical application of immunogenic cell death-related genes in hepatocellular carcinoma
BMC Cancer (2023)
-
Ultrasound-responsive low-dose doxorubicin liposomes trigger mitochondrial DNA release and activate cGAS-STING-mediated antitumour immunity
Nature Communications (2023)