Key Points
-
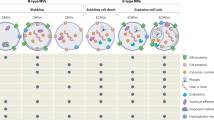
Outer membrane vesicles (OMVs) are bacterial nanoparticles that are naturally produced during bacterial growth both in vitro and in vivo. OMVs can interact with many host cell types — including mucosal epithelial cells, myeloid cells and cells distal to the site of OMV entry — and thus have a range of inflammatory outcomes.
-
Clinical studies have demonstrated the presence of OMVs in host tissues, suggesting that they have potentially pathogenic roles in various infectious diseases, particularly in those of a chronic nature. OMVs may also be important as previously unrecognized mediators of the inflammatory pathologies that accompany certain infectious diseases of idiopathic origin.
-
OMVs can enter non-phagocytic human epithelial cells via multiple mechanisms including lipid raft-dependent and lipid raft-independent endocytosis, in addition to dynamin-dependent and dynamin-independent mechanisms. When they are present inside cells, OMVs migrate to early endosomes and are detected by the host intracellular immune receptor nucleotide-binding oligomerization domain-containing protein 1 (NOD1), which results in the induction of autophagy and the generation of a pro-inflammatory response.
-
When OMVs are within epithelial cells, their protein cargo is processed and OMV peptides are released in exosomes. These peptide-laden exosomes can then be taken up by professional antigen-presenting cells and presented to T cells, resulting in the generation of antigen-specific adaptive immune responses.
-
In addition to their pro-inflammatory effects, OMVs can modulate or even suppress immune cell responses through their direct effects on host cells. Evidence is also emerging that OMVs produced by commensal bacteria may have roles in immune tolerance and other physiological functions of benefit to the host.
-
OMVs are highly stable, non-infectious and genetically tractable nanoparticles that contain the major immunogenic proteins of the parent bacterium and are able to elicit responses from both arms of the immune system, thus making them highly suited as vaccines and adjuvants.
Abstract
Gram-negative bacteria shed extracellular outer membrane vesicles (OMVs) during their normal growth both in vitro and in vivo. OMVs are spherical, bilayered membrane nanostructures that contain many components found within the parent bacterium. Until recently, OMVs were dismissed as a by-product of bacterial growth; however, findings within the past decade have revealed that both pathogenic and commensal bacteria can use OMVs to manipulate the host immune response. In this Review, we describe the mechanisms through which OMVs induce host pathology or immune tolerance, and we discuss the development of OMVs as innovative nanotechnologies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kulp, A. & Kuehn, M. J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 64, 163–184 (2010).
Chatterjee, S. N. & Das, J. Electron microscopic observations on the excretion of cell-wall material by Vibrio cholerae. J. General Microbiol. 49, 1–11 (1967).
DeVoe, I. W. & Gilchrist, J. E. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J. Exp. Med. 138, 1156–1167 (1973).
DeVoe, I. W. & Gilchrist, J. E. Pili on meningococci from primary cultures of nasopharyngeal carriers and cerebrospinal fluid of patients with acute disease. J. Exp. Med. 141, 297–305 (1975).
Schooling, S. R. & Beveridge, T. J. Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol. 188, 5945–5957 (2006).
Kadurugamuwa, J. L. & Beveridge, T. J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol. 177, 3998–4008 (1995).
Renelli, M., Matias, V., Lo, R. Y. & Beveridge, T. J. DNA-containing membrane vesicles of Pseudomonas aeruginosa PAO1 and their genetic transformation potential. Microbiology 150, 2161–2169 (2004).
Biller, S. J. et al. Bacterial vesicles in marine ecosystems. Science 343, 183–186 (2014).
Shen, Y. et al. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe 12, 509–520 (2012). This is the first identification of commensal OMVs having a protective and immunoregulatory role at the host mucosal surface, showing that the gut commensal B. fragilis releases OMVs with immunomodulatory and protective effects against colitis.
Craven, D. E. et al. Adherence of isolates of Neisseria meningitidis from patients and carriers to human buccal epithelial cells. J. Infect. Dis. 142, 556–568 (1980).
Stephens, D. S., Edwards, K. M., Morris, F. & McGee, Z. A. Pili and outer membrane appendages on Neisseria meningitidis in the cerebrospinal fluid of an infant. J. Infect. Dis. 146, 568 (1982).
Fiocca, R. et al. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J. Pathol. 188, 220–226 (1999).
Keenan, J. et al. A role for the bacterial outer membrane in the pathogenesis of Helicobacter pylori infection. FEMS Microbiol. Lett. 182, 259–264 (2000).
Vidakovics, M. L. et al. B cell activation by outer membrane vesicles — a novel virulence mechanism. PLoS Pathog. 6, e1000724 (2010).
Ren, D. et al. Characterization of extended co-culture of non-typeable Haemophilus influenzae with primary human respiratory tissues. Exp. Biol. Med. 237, 540–547 (2012).
Ellis, T. N. & Kuehn, M. J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 74, 81–94 (2010).
Ismail, S., Hampton, M. B. & Keenan, J. I. Helicobacter pylori outer membrane vesicles modulate proliferation and interleukin-8 production by gastric epithelial cells. Infect. Immun. 71, 5670–5675 (2003). This is one of the earliest reports showing that OMVs can induce IL-8 production in human epithelial cells.
Bauman, S. J. & Kuehn, M. J. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 8, 2400–2408 (2006).
Galka, F. et al. Proteomic characterization of the whole secretome of Legionella pneumophila and functional analysis of outer membrane vesicles. Infect. Immun. 76, 1825–1836 (2008).
Lee, J. C. et al. Klebsiella pneumoniae secretes outer membrane vesicles that induce the innate immune response. FEMS Microbiol. Lett. 331, 17–24 (2012).
Jun, S. H. et al. Acinetobacter baumannii outer membrane vesicles elicit a potent innate immune response via membrane proteins. PLoS ONE 8, e71751 (2013).
Kaparakis, M. et al. Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell. Microbiol. 12, 372–385 (2010). This study shows that NOD1 detects peptidoglycan contained within OMVs and that this is essential for the induction of innate and OMV-specific adaptive immune responses in vivo.
Kuehn, M. J. & Kesty, N. C. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 19, 2645–2655 (2005).
Soderblom, T. et al. Effects of the Escherichia coli toxin cytolysin A on mucosal immunostimulation via epithelial Ca2+ signalling and Toll-like receptor 4. Cell. Microbiol. 7, 779–788 (2005).
Zhao, K., Deng, X., He, C., Yue, B. & Wu, M. Pseudomonas aeruginosa outer membrane vesicles modulate host immune responses by targeting the Toll-like receptor 4 signaling pathway. Infect. Immun. 81, 4509–4518 (2013).
Allison, C. C., Kufer, T. A., Kremmer, E., Kaparakis, M. & Ferrero, R. L. Helicobacter pylori induces MAPK phosphorylation and AP-1 activation via a NOD1-dependent mechanism. J. Immunol. 183, 8099–8109 (2009).
Sharpe, S. W., Kuehn, M. J. & Mason, K. M. Elicitation of epithelial cell-derived immune effectors by outer membrane vesicles of nontypeable Haemophilus influenzae. Infection Immun. 79, 4361–4369 (2011).
Grubman, A. et al. The innate immune molecule, NOD1, regulates direct killing of Helicobacter pylori by antimicrobial peptides. Cell. Microbiol. 12, 626–639 (2010).
Bauer, B. et al. The Helicobacter pylori virulence effector CagA abrogates human β-defensin 3 expression via inactivation of EGFR signaling. Cell Host Microbe 11, 576–586 (2012).
Elmi, A. et al. Campylobacter jejuni outer membrane vesicles play an important role in bacterial interactions with human intestinal epithelial cells. Infect. Immun. 80, 4089–4098 (2012).
Nourshargh, S. et al. A comparative study of the neutrophil stimulatory activity in vitro and pro-inflammatory properties in vivo of 72 amino acid and 77 amino acid IL-8. J. Immunol. 148, 106–111 (1992).
Mikolajczyk-Pawlinska, J., Travis, J. & Potempa, J. Modulation of interleukin-8 activity by gingipains from Porphyromonas gingivalis: implications for pathogenicity of periodontal disease. FEBS Lett. 440, 282–286 (1998).
Pollak, C. N., Delpino, M. V., Fossati, C. A. & Baldi, P. C. Outer membrane vesicles from Brucella abortus promote bacterial internalization by human monocytes and modulate their innate immune response. PLoS ONE 7, e50214 (2012).
Mirlashari, M. R., Høiby, E. A., Holst, J. & Lyberg, T. Outer membrane vesicles from Neisseria meningitidis: effects on cytokine production in human whole blood. Cytokine 13, 91–97 (2001).
Chi, B., Qi, M. & Kuramitsu, H. K. Role of dentilisin in Treponema denticola epithelial cell layer penetration. Res. Microbiol. 154, 637–643 (2003).
Nakao, R. et al. Effect of Porphyromonas gingivalis outer membrane vesicles on gingipain-mediated detachment of cultured oral epithelial cells and immune responses. Microbes Infect. 16, 6–16 (2014).
Lapinet, J. A., Scapini, P., Calzetti, F., Perez, O. & Cassatella, M. A. Gene expression and production of tumor necrosis factor alpha, interleukin-1β (IL-1β), IL-8, macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and gamma interferon-inducible protein 10 by human neutrophils stimulated with group B meningococcal outer membrane vesicles. Infect. Immun. 68, 6917–6923 (2000).
Davis, J. M., Carvalho, H. M., Rasmussen, S. B. & O'Brien, A. D. Cytotoxic necrotizing factor type 1 delivered by outer membrane vesicles of uropathogenic Escherichia coli attenuates polymorphonuclear leukocyte antimicrobial activity and chemotaxis. Infect. Immun. 74, 4401–4408 (2006).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Lappann, M. et al. In vitro resistance mechanisms of Neisseria meningitidis against neutrophil extracellular traps. Mol. Microbiol. 89, 433–449 (2013).
Hellenbrand, K. M., Forsythe, K. M., Rivera-Rivas, J. J., Czuprynski, C. J. & Aulik, N. A. Histophilus somni causes extracellular trap formation by bovine neutrophils and macrophages. Microb. Pathog. 54, 67–75 (2013).
Jager, J. et al. Human lung tissue explants reveal novel interactions during Legionella pneumophila infections. Infect. Immun. 82, 275–285 (2013).
Tavano, R. et al. The membrane expression of Neisseria meningitidis adhesin A (NadA) increases the proimmune effects of MenB OMVs on human macrophages, compared with NadA- OMVs, without further stimulating their proinflammatory activity on circulating monocytes. J. Leukoc. Biol. 86, 143–153 (2009).
Winter, J., Letley, D., Rhead, J., Atherton, J. & Robinson, K. Helicobacter pylori membrane vesicles stimulate innate pro- and anti-inflammatory responses and induce apoptosis in Jurkat T cells. Infect. Immun. 82, 1372–1381 (2014).
Alaniz, R. C., Deatherage, B. L., Lara, J. C. & Cookson, B. T. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol. 179, 7692–7701 (2007). This study identifies the ability of OMVs derived from Salmonella spp. to induce activation of DCs and promote the development of protective B cell and T cell responses in vivo.
Imayoshi, R., Cho, T. & Kaminishi, H. NO production in RAW264 cells stimulated with Porphyromonas gingivalis extracellular vesicles. Oral Dis. 17, 83–89 (2011).
Mirlashari, M. R., Hoiby, E. A., Holst, J. & Lyberg, T. Outer membrane vesicles from Neisseria meningitidis: effects on tissue factor and plasminogen activator inhibitor-2 production in human monocytes. Thromb. Res. 102, 375–380 (2001).
Duncan, L., Yoshioka, M., Chandad, F. & Grenier, D. Loss of lipopolysaccharide receptor CD14 from the surface of human macrophage-like cells mediated by Porphyromonas gingivalis outer membrane vesicles. Microb. Pathog. 36, 319–325 (2004).
Durand, V. et al. Role of lipopolysaccharide in the induction of type I interferon-dependent cross-priming and IL-10 production in mice by meningococcal outer membrane vesicles. Vaccine 27, 1912–1922 (2009).
Schultz, H., Hume, J., Zhang de, S., Gioannini, T. L. & Weiss, J. P. A novel role for the bactericidal/permeability increasing protein in interactions of gram-negative bacterial outer membrane blebs with dendritic cells. J. Immunol. 179, 2477–2484 (2007).
Weiss, J. et al. Human bactericidal/permeability-increasing protein and a recombinant NH2-terminal fragment cause killing of serum-resistant gram-negative bacteria in whole blood and inhibit tumor necrosis factor release induced by the bacteria. J. Clin. Invest. 90, 1122–1130 (1992).
Soult, M. C. et al. Outer membrane vesicles from pathogenic bacteria initiate an inflammatory response in human endothelial cells. J. Surg. Res. 184, 458–466 (2013).
Soult, M. C., Dobrydneva, Y., Wahab, K. H., Britt, L. D. & Sullivan, C. J. Outer membrane vesicles alter inflammation and coagulation mediators. J. Surg. Res. 192, 134–142 (2014).
Kim, J. H. et al. Outer membrane vesicles derived from Escherichia coli up-regulate expression of endothelial cell adhesion molecules in vitro and in vivo. PLoS ONE 8, e59276 (2013).
Srisatjaluk, R., Kotwal, G. J., Hunt, L. A. & Justus, D. E. Modulation of γ interferon-induced major histocompatibility complex class II gene expression by Porphyromonas gingivalis membrane vesicles. Infect. Immun. 70, 1185–1192 (2002).
Mirlashari, M. R., Hagberg, I. A. & Lyberg, T. Platelet–platelet and platelet–leukocyte interactions induced by outer membrane vesicles from N. meningitidis. Platelets 13, 91–99 (2002).
Sharma, A. et al. Porphyromonas gingivalis platelet aggregation activity: outer membrane vesicles are potent activators of murine platelets. Oral Microbiol. Immunol. 15, 393–396 (2000).
Maldonado, R., Wei, R., Kachlany, S. C., Kazi, M. & Balashova, N. V. Cytotoxic effects of Kingella kingae outer membrane vesicles on human cells. Microb. Pathog. 51, 22–30 (2011).
Schaar, V. et al. Multicomponent Moraxella catarrhalis outer membrane vesicles induce an inflammatory response and are internalized by human epithelial cells. Cell. Microbiol. 13, 432–449 (2010).
Kesty, N. C., Mason, K. M., Reedy, M., Miller, S. E. & Kuehn, M. J. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 23, 4538–4549 (2004). This is the earliest report identifying the ability of OMVs to enter non-phagocytic host epithelial cells in a lipid raft-dependent manner.
Bauman, S. J. & Kuehn, M. J. Pseudomonas aeruginosa vesicles associate with and are internalized by human lung epithelial cells. BMC Microbiol. 9, 26 (2009).
Furuta, N. et al. Porphyromonas gingivalis outer membrane vesicles enter human epithelial cells via an endocytic pathway and are sorted to lysosomal compartments. Infect. Immun. 77, 4187–4196 (2009).
Olofsson, A., Nygard Skalman, L., Obi, I., Lundmark, R. & Arnqvist, A. Uptake of Helicobacter pylori vesicles is facilitated by clathrin-dependent and clathrin-independent endocytic pathways. mBio 5, e00979-14 (2014).
Bomberger, J. M. et al. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 5, e1000382 (2009).
Rompikuntal, P. K. et al. Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans. Infect. Immun. 80, 31–42 (2012).
Bielaszewska, M. et al. Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis. PLoS Pathog. 9, e1003797 (2013).
Parker, H., Chitcholtan, K., Hampton, M. B. & Keenan, J. I. Uptake of Helicobacter pylori outer membrane vesicles by gastric epithelial cells. Infect. Immun. 78, 5054–5061 (2010).
Crowley, J. T. et al. Lipid exchange between Borrelia burgdorferi and host cells. PLoS Pathog. 9, e1003109 (2013).
Bielig, H. et al. NOD-like receptor activation by outer membrane vesicles from Vibrio cholerae non-O1 non-O139 strains is modulated by the quorum-sensing regulator HapR. Infect. Immun. 79, 1418–1427 (2011).
Chatterjee, D. & Chaudhuri, K. Vibrio cholerae O395 outer membrane vesicles modulate intestinal epithelial cells in a NOD1 protein-dependent manner and induce dendritic cell-mediated Th2/Th17 cell responses. J. Biol. Chem. 288, 4299–4309 (2013).
Thay, B., Damm, A., Kufer, T. A., Wai, S. N. & Oscarsson, J. Aggregatibacter actinomycetemcomitans outer membrane vesicles are internalized in human host cells and trigger NOD1- and NOD2-dependent NF-κB activation. Infect. Immun. 82, 4034–4046 (2014).
Irving, A. T. et al. The immune receptor NOD1 and kinase RIP2 interact with bacterial peptidoglycan on early endosomes to promote autophagy and inflammatory signaling. Cell Host Microbe 15, 623–635 (2014). This is the first report showing that when they are present inside cells, OMVs migrate to early endosomes, where they are detected by NOD1 and subsequently degraded by NOD1-dependent autophagy, resulting in the initiation of an inflammatory response.
Chitcholtan, K., Hampton, M. B. & Keenan, J. I. Outer membrane vesicles enhance the carcinogenic potential of Helicobacter pylori. Carcinogenesis 29, 2400–2405 (2008).
Guidi, R. et al. Salmonella enterica delivers its genotoxin through outer membrane vesicles secreted from infected cells. Cell. Microbiol. 15, 2034–2050 (2013). This study shows that Salmonella enterica -infected host cells secrete OMVs containing cytolethal distending toxin, which is genotoxic, and that they subsequently enter neighbouring cells, causing secondary bystander effects.
Tyrer, P. C., Frizelle, F. A. & Keenan, J. I. Escherichia coli-derived outer membrane vesicles are genotoxic to human enterocyte-like cells. Infect. Agents Cancer 9, 2 (2014).
Etchart, N. et al. Intranasal immunisation with inactivated RSV and bacterial adjuvants induces mucosal protection and abrogates eosinophilia upon challenge. Eur. J. Immunol. 36, 1136–1144 (2006).
Fantappiè, L. et al. Antibody-mediated immunity induced by engineered Escherichia coli OMVs carrying heterologous antigens in their lumen. J. Extracell. Vesicles 3, 24015 (2014).
Guthrie, T. et al. Local and systemic antibody responses in mice immunized intranasally with native and detergent-extracted outer membrane vesicles from Neisseria meningitidis. Infect. Immun. 72, 2528–2537 (2004).
Huang, W. et al. Immunization against multidrug-resistant Acinetobacter baumannii effectively protects mice in both pneumonia and sepsis models. PLoS ONE 9, e100727 (2014).
Keenan, J. et al. Immune response to an 18-kilodalton outer membrane antigen identifies lipoprotein 20 as a Helicobacter pylori vaccine candidate. Infect. Immun. 68, 3337–3343 (2000).
Schild, S., Nelson, E. J. & Camilli, A. Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice. Infect. Immun. 76, 4554–4563 (2008).
Davenport, V. et al. Mucosal immunity in healthy adults after parenteral vaccination with outer-membrane vesicles from Neisseria meningitidis serogroup B. J. Infect. Dis. 198, 731–740 (2008).
Aghasadeghi, M. R. et al. Application of outer membrane vesicle of Neisseria meningitidis serogroup B as a new adjuvant to induce strongly Th1-oriented responses against HIV-1. Curr. HIV Res. 9, 630–635 (2011).
Camacho, A. I., Irache, J. M., de Souza, J., Sánchez-Gómez, S. & Gamazo, C. Nanoparticle-based vaccine for mucosal protection against Shigella flexneri in mice. Vaccine 31, 3288–3294 (2013).
Roier, S. et al. Intranasal immunization with nontypeable Haemophilus influenzae outer membrane vesicles induces cross-protective immunity in mice. PLoS ONE 7, e42664 (2012).
Roier, S. et al. Immunogenicity of Pasteurella multocida and Mannheimia haemolytica outer membrane vesicles. Int. J. Med. Microbiol. 303, 247–256 (2013).
Nieves, W. et al. A naturally derived outer-membrane vesicle vaccine protects against lethal pulmonary Burkholderia pseudomallei infection. Vaccine 29, 8381–8389 (2011).
Kim, O. Y. et al. Immunization with Escherichia coli outer membrane vesicles protects bacteria-induced lethality via Th1 and Th17 cell responses. J. Immunol. 190, 4092–4102 (2013).
Perez, O. et al. Immune response induction and new effector mechanisms possibly involved in protection conferred by the Cuban anti-meningococcal BC vaccine. Infect. Immun. 69, 4502–4508 (2001).
Turner, L. et al. Increased outer membrane vesicle formation in a Helicobacter pylori tolB mutant. Helicobacter http://dx.doi.org/10.1111/hel.12196 (2015).
Lee, H. S. et al. Neisserial outer membrane vesicles bind the coinhibitory receptor carcinoembryonic antigen-related cellular adhesion molecule 1 and suppress CD4+ T lymphocyte function. Infect. Immun. 75, 4449–4455 (2007).
Youssef, A. R. et al. Opa+ and Opa− isolates of Neisseria meningitidis and Neisseria gonorrhoeae induce sustained proliferative responses in human CD4+ T cells. Infection Immun. 77, 5170–5180 (2009).
Deknuydt, F., Nordstrom, T. & Riesbeck, K. Diversion of the host humoral response: a novel virulence mechanism of Haemophilus influenzae mediated via outer membrane vesicles. J. Leukoc. Biol. 95, 983–991 (2014).
Vaughan, A. T. et al. Neisseria lactamica selectively induces mitogenic proliferation of the naive B cell pool via cell surface Ig. J. Immunol. 185, 3652–3660 (2010).
Vaughan, A. T., Gorringe, A., Davenport, V., Williams, N. A. & Heyderman, R. S. Absence of mucosal immunity in the human upper respiratory tract to the commensal bacteria Neisseria lactamica but not pathogenic Neisseria meningitidis during the peak age of nasopharyngeal carriage. J. Immunol. 182, 2231–2240 (2009).
Mallegol, J. et al. T84-intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology 132, 1866–1876 (2007).
Van Niel, G. et al. Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut 52, 1690–1697 (2003).
Giri, P. K. & Schorey, J. S. Exosomes derived from M. bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS ONE 3, e2461 (2008).
Veith, P. D. et al. Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J. Proteome Res. 13, 2420–2432 (2014).
Rolhion, N. et al. Abnormally expressed ER stress response chaperone Gp96 in CD favours adherent-invasive Escherichia coli invasion. Gut 59, 1355–1362 (2010). This study identifies the ability of OMVs to facilitate bacterial colonization, potentially contributing to further pathology in patients with Crohn disease.
Gregg, C. R., Melly, M. A., Hellerqvist, C. G., Coniglio, J. G. & McGee, Z. A. Toxic activity of purified lipopolysaccharide of Neisseria gonorrhoeae for human fallopian tube mucosa. J. Infect. Dis. 143, 432–439 (1981).
Namork, E. & Brandtzaeg, P. Fatal meningococcal septicaemia with “blebbing” meningococcus. Lancet 360, 1741 (2002).
Shah, B. et al. Circulating bacterial membrane vesicles cause sepsis in rats. Shock 37, 621–628 (2012).
Kuramitsu, H. K., Qi, M., Kang, I. C. & Chen, W. Role for periodontal bacteria in cardiovascular diseases. Ann. Periodontol. 6, 41–47 (2001).
Halhoul, N. & Colvin, J. R. The ultrastructure of bacterial plaque attached to the gingiva of man. Arch. Oral Biol. 20, 115–118 (1975).
Brandtzaeg, P. et al. Meningococcal endotoxin in lethal septic shock plasma studied by gas chromatography, mass-spectrometry, ultracentrifugation, and electron microscopy. J. Clin. Invest. 89, 816–823 (1992).
Srisatjaluk, R., Doyle, R. J. & Justus, D. E. Outer membrane vesicles of Porphyromonas gingivalis inhibit IFN-γ-mediated MHC class II expression by human vascular endothelial cells. Microb. Pathog. 27, 81–91 (1999).
Slocum, C. et al. Distinct lipid A moieties contribute to pathogen-induced site-specific vascular inflammation. PLoS Pathog. 10, e1004215 (2014).
Stentz, R. et al. A bacterial homolog of a eukaryotic inositol phosphate signaling enzyme mediates cross-kingdom dialog in the mammalian gut. Cell Rep. 6, 646–656 (2014).
Oertli, M. et al. Helicobacter pylori γ-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc. Natl Acad. Sci. USA 110, 3047–3052 (2013).
Arigita, C. et al. Stability of mono- and trivalent meningococcal outer membrane vesicle vaccines. Vaccine 22, 629–642 (2004).
Baker, J. L., Chen, L., Rosenthal, J. A., Putnam, D. & DeLisa, M. P. Microbial biosynthesis of designer outer membrane vesicles. Curr. Opin. Biotechnol. 29, 76–84 (2014).
Tani, C. et al. Quantification by LC-MSE of outer membrane vesicle proteins of the Bexsero® vaccine. Vaccine 32, 1273–1279 (2014).
Ellis, T. N., Leiman, S. A. & Kuehn, M. J. Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect. Immun. 78, 3822–3831 (2010).
Sanders, H. & Feavers, I. M. Adjuvant properties of meningococcal outer membrane vesicles and the use of adjuvants in Neisseria meningitidis protein vaccines. Expert Rev. Vaccines 10, 323–334 (2011).
Chen, D. J. et al. Delivery of foreign antigens by engineered outer membrane vesicle vaccines. Proc. Natl Acad. Sci. USA 107, 3099–3104 (2010). This paper provides proof-of-concept evidence for the use of OMVs as a vaccine platform, reporting that adjuvant-free immunization with E. coli OMVs expressing green fluorescent protein (GFP) elicited strong GFP-specific antibody responses in mice.
Daleke-Schermerhorn, M. H. et al. Decoration of outer membrane vesicles with multiple antigens by using an autotransporter approach. Appl. Environ. Microbiol. 80, 5854–5865 (2014).
Schild, S., Nelson, E. J., Bishop, A. L. & Camilli, A. Characterization of Vibrio cholerae outer membrane vesicles as a candidate vaccine for cholera. Infect. Immun. 77, 472–484 (2009).
Asensio, C. J. et al. Outer membrane vesicles obtained from Bordetella pertussis Tohama expressing the lipid A deacylase PagL as a novel acellular vaccine candidate. Vaccine 29, 1649–1656 (2011).
Lee, D. H. et al. Adjuvant effect of bacterial outer membrane vesicles with penta-acylated lipopolysaccharide on antigen-specific T cell priming. Vaccine 29, 8293–8301 (2011).
Weynants, V. et al. Genetically modified L3,7 and L2 lipooligosaccharides from Neisseria meningitidis serogroup B confer a broad cross-bactericidal response. Infect. Immun. 77, 2084–2093 (2009).
Oster, P. et al. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine 23, 2191–2196 (2005).
Vesikari, T. et al. Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet 381, 825–835 (2013).
Andrews, S. M. & Pollard, A. J. A vaccine against serogroup B Neisseria meningitidis: dealing with uncertainty. Lancet Infect. Dis. 14, 426–434 (2014).
Rivera, J. et al. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc. Natl Acad. Sci. USA 107, 19002–19007 (2010).
Prados-Rosales, R. et al. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J. Clin. Invest. 121, 1471–1483 (2011).
Kim, Y. S. et al. Extracellular vesicles, especially derived from Gram-negative bacteria, in indoor dust induce neutrophilic pulmonary inflammation associated with both Th1 and Th17 cell responses. Clin. Exp. Allergy 43, 443–454 (2013).
Kamada, N., Seo, S. U., Chen, G. Y. & Nunez, G. Role of the gut microbiota in immunity and inflammatory disease. Nature Rev. Immunol. 13, 321–335 (2013).
Gujrati, V. et al. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano 8, 1525–1537 (2014). This is the first report that harnesses OMV-based technology to deliver targeted and effective cancer therapy agents to animals.
Whitchurch, C. B., Tolker-Nielsen, T., Ragas, P. C. & Mattick, J. S. Extracellular DNA required for bacterial biofilm formation. Science 295, 1487 (2002).
Keenan, J. I., Davis, K. A., Beaugie, C. R., McGovern, J. J. & Moran, A. P. Alterations in Helicobacter pylori outer membrane and outer membrane vesicle-associated lipopolysaccharides under iron-limiting growth conditions. Innate Immun. 14, 279–290 (2008).
Dorward, D. W., Garon, C. F. & Judd, R. C. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J. Bacteriol. 171, 2499–2505 (1989).
Bernadac, A., Gavioli, M., Lazzaroni, J. C., Raina, S. & Lloubes, R. Escherichia coli tol-pal mutants form outer membrane vesicles. J. Bacteriol. 180, 4872–4878 (1998).
Macdonald, I. A. & Kuehn, M. J. Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa. J. Bacteriol. 195, 2971–2981 (2013).
McBroom, A. J. & Kuehn, M. J. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 63, 545–558 (2007).
Acknowledgements
The work was supported by the Australian Research Council (M.K.-L.), the National Health and Medical Research Council (NHMRC; R.L.F.) and the Victorian Government's Operational Infrastructure Support Program to the MIMR-PHI Institute of Medical Research. R.L.F. is an NHMRC Senior Research Fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Pattern recognition receptors
-
(PRRs). Germline-encoded immune receptors that detect conserved microorganism-associated molecular patterns and function to initiate immune signalling and the development of a pro-inflammatory innate immune response.
- Tol–Pal system
-
A system encoded by a cluster comprising 5–7 genes that is conserved across most Gram-negative microorganisms. These genes encode inner membrane and periplasmic proteins, as well as a peptidoglycan-associated protein. Disruption of these genes may result in phenotypic changes, including changes in cell morphology.
- Microorganism-associated molecular patterns
-
(MAMPs). Conserved microbial determinants that are detected by host pattern recognition receptors, resulting in the induction of immune signalling in the host.
- Nucleotide-binding oligomerization domain-containing protein 1
-
(NOD1). A host cytoplasmic innate immune pattern recognition receptor that specifically detects a conserved structural motif within the peptidoglycan of almost all Gram-negative bacteria.
- Human β-defensins
-
Small, cationic antimicrobial peptides that are produced by epithelial cells in response to pro-inflammatory cytokines or bacteria.
- Neutrophil extracellular traps
-
(NETs). Structures containing DNA, antimicrobial peptides and histones that form extracellular fibres that trap and degrade virulence factors, in addition to killing extracellular pathogens.
- Lipid rafts
-
Cholesterol- and sphingolipid-rich microdomains located on the surface of the plasma membranes of host cells that can be used by outer membrane vesicles as a portal of entry.
- Autophagy
-
A host intracellular degradation process that can be induced by NOD1-mediated detection of Gram-negative bacterial peptidoglycan.
- Exosomes
-
Small extracellular vesicles that are derived from multivesicular bodies or from the plasma membrane of eukaryotic cells.
- Adjuvant
-
An agent or compound that when administered with an antigenic preparation (for example, a vaccine), provides stronger and more long-lasting immune responses to the antigen.
Rights and permissions
About this article
Cite this article
Kaparakis-Liaskos, M., Ferrero, R. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol 15, 375–387 (2015). https://doi.org/10.1038/nri3837
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3837
This article is cited by
-
Exploiting bacterial-origin immunostimulants for improved vaccination and immunotherapy: current insights and future directions
Cell & Bioscience (2024)
-
Prokaryotic microvesicles Ortholog of eukaryotic extracellular vesicles in biomedical fields
Cell Communication and Signaling (2024)
-
Bacterial c-di-GMP signaling gene affects mussel larval metamorphosis through outer membrane vesicles and lipopolysaccharides
npj Biofilms and Microbiomes (2024)
-
Maternal microbiota communicates with the fetus through microbiota-derived extracellular vesicles
Microbiome (2023)
-
Detoxified synthetic bacterial membrane vesicles as a vaccine platform against bacteria and SARS-CoV-2
Journal of Nanobiotechnology (2023)