Key Points
-
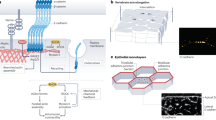
Adherens junctions (AJs) meet the dual challenge of maintaining tissue architecture and facilitating cell movement during tissue development and renewal.
-
Across animal species, classic cadherins display diversity in the structure of their extracellular regions but share a conserved cytoplasmic tail and a common tendency to form clusters at the plasma membrane.
-
The cytoplasmic tail of classic cadherin binds to the catenins, which mediate links to cytoskeletal networks as well as exocytotic and endocytic machinery.
-
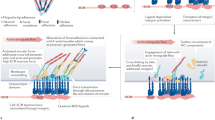
Crosstalk between cadherin–catenin clusters and actin regulators directs AJ assembly from initial cell–cell contacts.
-
Links between cadherin–catenin clusters and microtubules organize epithelial cells more globally.
-
Regulated endocytosis of cadherin–catenin clusters facilitates AJ remodelling.
-
The effects of cytoskeletal or endocytic regulation of AJs on overall tissue structure depends on whether the regulation occurs locally, at a subset of cell–cell contacts of individual cells, or globally, at all cell–cell contacts.
Abstract
How adhesive interactions between cells generate and maintain animal tissue structure remains one of the most challenging and long-standing questions in cell and developmental biology. Adherens junctions (AJs) and the cadherin–catenin complexes at their core are therefore the subjects of intense research. Recent work has greatly advanced our understanding of the molecular organization of AJs and how cadherin–catenin complexes engage actin, microtubules and the endocytic machinery. As a result, we have gained important insights into the molecular mechanisms of tissue morphogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gumbiner, B. M. Regulation of cadherin-mediated adhesion in morphogenesis. Nature Rev. Mol. Cell Biol. 6, 622–634 (2005).
Halbleib, J. M. & Nelson, W. J. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 20, 3199–3214 (2006).
Nishimura, T. & Takeichi, M. Remodeling of the adherens junctions during morphogenesis. Curr. Top. Dev. Biol. 89, 33–54 (2009).
Farquhar, M. G. & Palade, G. E. Junctional complexes in various epithelia. J. Cell Biol. 17, 375–412 (1963). The first clear morphological descriptions of AJs and other epithelial junctions by electron microscopy in mammalian tissues.
Hirokawa, N. & Heuser, J. E. Quick-freeze, deep-etch visualization of the cytoskeleton beneath surface differentiations of intestinal epithelial cells. J. Cell Biol. 91, 399–409 (1981).
Miyaguchi, K. Ultrastructure of the zonula adherens revealed by rapid-freeze deep-etching. J. Struct. Biol. 132, 169–178 (2000).
Takeichi, M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251, 1451–1455 (1991).
Pokutta, S. & Weis, W. I. Structure and mechanism of cadherins and catenins in cell–cell contacts. Annu. Rev. Cell Dev. Biol. 23, 237–261 (2007).
Perez-Moreno, M. & Fuchs, E. Catenins: keeping cells from getting their signals crossed. Dev. Cell 11, 601–612 (2006).
Franke, W. W. Discovering the molecular components of intercellular junctions — a historical view. Cold Spring Harbor Perspect. Biol. 1, a003061 (2009).
Berx, G. & van Roy, F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harbor Perspect. Biol. 1, a003129 (2009).
Bonazzi, M., Lecuit, M. & Cossart, P. Listeria monocytogenes internalin and E-cadherin: from bench to bedside. Cold Spring Harbor Perspect. Biol. 1, a003087 (2009).
Grell, K. G. & Ruthmann, A. Placozoa. In Microscopic Anatomy of Invertebrates, Placozoa, Porifera, Cnidaria and Ctenophora. (eds F.H. & J.W.) (Wiley-Liss, New York, 1991).
Kraus, Y. & Technau, U. Gastrulation in the sea anemone Nematostella vectensis occurs by invagination and immigration: an ultrastructural study. Dev. Genes Evol. 216, 119–132 (2006).
Chapman, J. A. et al. The dynamic genome of Hydra. Nature 464, 592–596 (2010).
Srivastava, M. et al. The Trichoplax genome and the nature of placozoans. Nature 454, 955–960 (2008).
Abedin, M. & King, N. The premetazoan ancestry of cadherins. Science 319, 946–948 (2008).
Grimson, M. J. et al. Adherens junctions and β-catenin-mediated cell signalling in a non-metazoan organism. Nature 408, 727–731 (2000). Identifies junctional complexes with AJ morphology in an organism without classic cadherins
Oda, H., Tagawa, K. & Akiyama-Oda, Y. Diversification of epithelial adherens junctions with independent reductive changes in cadherin form: identification of potential molecular synapomorphies among bilaterians. Evol. Dev. 7, 376–389 (2005). A reconstruction of the evolution of classic cadherins during animal evolution.
Hulpiau, P. & van Roy, F. Molecular evolution of the cadherin superfamily. Int. J. Biochem. Cell Biol. 41, 349–369 (2009).
Iwai, Y. et al. Axon patterning requires DN-cadherin, a novel neuronal adhesion receptor, in the Drosophila embryonic CNS. Neuron 19, 77–89 (1997).
Miller, J. R. & McClay, D. R. Characterization of the role of cadherin in regulating cell adhesion during sea urchin development. Dev. Biol. 192, 323–339 (1997).
Broadbent, I. D. & Pettitt, J. The C. elegans hmr-1 gene can encode a neuronal classic cadherin involved in the regulation of axon fasciculation. Curr. Biol. 12, 59–63 (2002).
Tanabe, K., Takeichi, M. & Nakagawa, S. Identification of a nonchordate-type classic cadherin in vertebrates: chicken Hz-cadherin is expressed in horizontal cells of the neural retina and contains a nonchordate-specific domain complex. Dev. Dyn. 229, 899–906 (2004).
Oda, H., Uemura, T., Harada, Y., Iwai, Y. & Takeichi, M. A Drosophila homolog of cadherin associated with Armadillo and essential for embryonic cell-cell adhesion. Dev. Biol. 165, 716–726 (1994).
Oda, H., Akiyama-Oda, Y. & Zhang, S. Two classic cadherin-related molecules with no cadherin extracellular repeats in the cephalochordate amphioxus: distinct adhesive specificities and possible involvement in the development of multicell-layered structures. J. Cell Sci. 117, 2757–2767 (2004).
Garrod, D. & Chidgey, M. Desmosome structure, composition and function. Biochim. Biophys. Acta 1778, 572–587 (2008).
Leckband, D. & Prakasam, A. Mechanism and dynamics of cadherin adhesion. Annu. Rev. Biomed. Eng. 8, 259–287 (2006).
Tsukasaki, Y. et al. Role of multiple bonds between the single cell adhesion molecules, nectin and cadherin, revealed by high sensitive force measurements. J. Mol. Biol. 367, 996–1006 (2007).
Kovacs, E. M. & Yap, A. S. Cell–cell contact: cooperating clusters of actin and cadherin. Curr. Biol. 18, R667–R669 (2008).
Troyanovsky, S. Cadherin dimers in cell–cell adhesion. Eur. J. Cell Biol. 84, 225–233 (2005).
He, W., Cowin, P. & Stokes, D. L. Untangling desmosomal knots with electron tomography. Science 302, 109–113 (2003).
Al-Amoudi, A., Diez, D. C., Betts, M. J. & Frangakis, A. S. The molecular architecture of cadherins in native epidermal desmosomes. Nature 450, 832–837 (2007). The alignment of the atomic structure of a classic cadherin extracellular domain to electron tomographic reconstructions of fully packed, cadherin-based intercellular junctions.
Owen, G. R., Acehan, D., Derr, K. D., Rice, W. J. & Stokes, D. L. Cryoelectron tomography of isolated desmosomes. Biochem. Soc. Trans. 36, 173–179 (2008).
McGill, M. A., McKinley, R. F. & Harris, T. J. Independent cadherin–catenin and Bazooka clusters interact to assemble adherens junctions. J. Cell Biol. 185, 787–796 (2009).
Cavey, M., Rauzi, M., Lenne, P. F. & Lecuit, T. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature 453, 751–756 (2008). Reveals that AJs are comprised of dispersed cadherin subclusters.
Xu, W. & Kimelman, D. Mechanistic insights from structural studies of β-catenin and its binding partners. J. Cell Sci. 120, 3337–3344 (2007).
Gavert, N. & Ben-Ze'ev, A. β-Catenin signaling in biological control and cancer. J. Cell Biochem. 102, 820–828 (2007).
Reynolds, A. B. p120-catenin: past and present. Biochim. Biophys. Acta 1773, 2–7 (2007).
McCrea, P. D. & Park, J. I. Developmental functions of the p120-catenin sub-family. Biochim. Biophys. Acta 1773, 17–33 (2007).
Benjamin, J. M. & Nelson, W. J. Bench to bedside and back again: molecular mechanisms of α-catenin function and roles in tumorigenesis. Semin. Cancer Biol. 18, 53–64 (2008).
Kobielak, A. & Fuchs, E. α-Catenin: at the junction of intercellular adhesion and actin dynamics. Nature Rev. Mol. Cell Biol. 5, 614–625 (2004).
Huber, A. H., Stewart, D. B., Laurents, D. V., Nelson, W. J. & Weis, W. I. The cadherin cytoplasmic domain is unstructured in the absence of β-catenin. A possible mechanism for regulating cadherin turnover. J. Biol. Chem. 276, 12301–12309 (2001).
Chen, Y. T., Stewart, D. B. & Nelson, W. J. Coupling assembly of the E-cadherin/β-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J. Cell Biol. 144, 687–699 (1999).
Lock, J. G. & Stow, J. L. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol. Biol. Cell 16, 1744–1755 (2005).
Langevin, J. et al. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-cadherin trafficking from recycling endosomes to the plasma membrane. Dev. Cell 9, 365–376 (2005).
Bajpai, S. et al. α-Catenin mediates initial E-cadherin-dependent cell–cell recognition and subsequent bond strengthening. Proc. Natl Acad. Sci. USA 105, 18331–18336 (2008).
Pacquelet, A. & Rorth, P. Regulatory mechanisms required for DE-cadherin function in cell migration and other types of adhesion. J. Cell Biol. 170, 803–812 (2005). Shows that the release of α-catenin from cadherin is not needed for several types of tissue morphogenesis.
Gorfinkiel, N. & Arias, A. M. Requirements for adherens junction components in the interaction between epithelial tissues during dorsal closure in Drosophila. J. Cell Sci. 120, 3289–3298 (2007).
Rhee, J., Buchan, T., Zukerberg, L., Lilien, J. & Balsamo, J. Cables links Robo-bound Abl kinase to N-cadherin-bound β-catenin to mediate Slit-induced modulation of adhesion and transcription. Nature Cell Biol. 9, 883–892 (2007).
Lilien, J. & Balsamo, J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of β-catenin. Curr. Opin. Cell Biol. 17, 459–465 (2005).
Delva, E. & Kowalczyk, A. P. Regulation of cadherin trafficking. Traffic 10, 259–267 (2009).
Ireton, R. C. et al. A novel role for p120 catenin in E-cadherin function. J. Cell Biol. 159, 465–476 (2002).
Davis, M. A., Ireton, R. C. & Reynolds, A. B. A core function for p120-catenin in cadherin turnover. J. Cell Biol. 163, 525–534 (2003).
Ishiyama, N. et al. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell–cell adhesion. Cell 141, 117–128 (2010).
Chen, X., Kojima, S., Borisy, G. G. & Green, K. J. p120 catenin associates with kinesin and facilitates the transport of cadherin–catenin complexes to intercellular junctions. J. Cell Biol. 163, 547–557 (2003).
Meng, W., Mushika, Y., Ichii, T. & Takeichi, M. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell–cell contacts. Cell 135, 948–959 (2008). Identifies a mechanism for linking microtubule minus ends to AJs.
Myster, S. H., Cavallo, R., Anderson, C. T., Fox, D. T. & Peifer, M. Drosophila p120catenin plays a supporting role in cell adhesion but is not an essential adherens junction component. J. Cell Biol. 160, 433–449 (2003).
Pettitt, J., Cox, E. A., Broadbent, I. D., Flett, A. & Hardin, J. The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin–catenin function during epidermal morphogenesis. J. Cell Biol. 162, 15–22 (2003).
Pacquelet, A., Lin, L. & Rorth, P. Binding site for p120/δ-catenin is not required for Drosophila E-cadherin function in vivo. J. Cell Biol. 160, 313–319 (2003).
Hirano, S., Kimoto, N., Shimoyama, Y., Hirohashi, S. & Takeichi, M. Identification of a neural α-catenin as a key regulator of cadherin function and multicellular organization. Cell 70, 293–301 (1992).
Rimm, D. L., Koslov, E. R., Kebriaei, P., Cianci, C. D. & Morrow, J. S. α1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc. Natl Acad. Sci. USA 92, 8813–8817 (1995).
Pokutta, S. & Weis, W. I. Structure of the dimerization and β-catenin-binding region of α-catenin. Mol. Cell 5, 533–543 (2000).
Yamada, S., Pokutta, S., Drees, F., Weis, W. I. & Nelson, W. J. Deconstructing the cadherin–catenin–actin complex. Cell 123, 889–901 (2005). Suggests that α-catenin cannot bind cadherin–β-catenin complexes and actin at the same time.
Drees, F., Pokutta, S., Yamada, S., Nelson, W. J. & Weis, W. I. α-Catenin is a molecular switch that binds E-cadherin–β-Catenin and regulates actin-filament assembly. Cell 123, 903–915 (2005).
Costa, M. et al. A putative catenin–cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J. Cell Biol. 141, 297–308 (1998).
Kametani, Y. & Takeichi, M. Basal-to-apical cadherin flow at cell junctions. Nature Cell Biol. 9, 92–98 (2007).
Abe, K. & Takeichi, M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc. Natl Acad. Sci. USA 105, 13–19 (2008).
Maul, R. S. et al. EPLIN regulates actin dynamics by cross-linking and stabilizing filaments. J. Cell Biol. 160, 399–407 (2003).
Sawyer, J. K., Harris, N. J., Slep, K. C., Gaul, U. & Peifer, M. The Drosophila afadin homologue Canoe regulates linkage of the actin cytoskeleton to adherens junctions during apical constriction. J. Cell Biol. 186, 57–73 (2009).
Kobielak, A., Pasolli, H. A. & Fuchs, E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nature Cell Biol. 6, 21–30 (2004).
McNeill, H., Ryan, T. A., Smith, S. J. & Nelson, W. J. Spatial and temporal dissection of immediate and early events following cadherin-mediated epithelial cell adhesion. J. Cell Biol. 120, 1217–1226 (1993). Provides some of the first descriptions of how AJs assemble as cells first come into contact.
Adams, C. L., Nelson, W. J. & Smith, S. J. Quantitative analysis of cadherin–catenin–actin reorganization during development of cell–cell adhesion. J. Cell Biol. 135, 1899–1911 (1996).
Adams, C. L., Chen, Y. T., Smith, S. J. & Nelson, W. J. Mechanisms of epithelial cell–cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin–green fluorescent protein. J. Cell Biol. 142, 1105–1119 (1998).
Vasioukhin, V. & Fuchs, E. Actin dynamics and cell–cell adhesion in epithelia. Curr. Opin. Cell Biol. 13, 76–84 (2001).
Vasioukhin, V., Bauer, C., Yin, M. & Fuchs, E. Directed actin polymerization is the driving force for epithelial cell–cell adhesion. Cell 100, 209–219 (2000).
Yonemura, S., Itoh, M., Nagafuchi, A. & Tsukita, S. Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J. Cell Sci. 108, 127–142 (1995).
Ivanov, A. I., Hunt, D., Utech, M., Nusrat, A. & Parkos, C. A. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol. Biol. Cell 16, 2636–2650 (2005).
Kishikawa, M., Suzuki, A. & Ohno, S. aPKC enables development of zonula adherens by antagonizing centripetal contraction of the circumferential actomyosin cables. J. Cell Sci. 121, 2481–2492 (2008).
Yamada, S. & Nelson, W. J. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell cell adhesion. J. Cell Biol. 178, 517–527 (2007). Reveals how Rho family GTPases are coordinated as AJs form and mature.
Zhang, J. et al. Actin at cell-cell junctions is composed of two dynamic and functional populations. J. Cell Sci. 118, 5549–5562 (2005).
Scott, J. A. et al. Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts. Mol. Biol. Cell 17, 1085–1095 (2006).
Verma, S. et al. Arp2/3 activity is necessary for efficient formation of E-cadherin adhesive contacts. J. Biol. Chem. 279, 34062–34070 (2004).
Kovacs, E. M., Goodwin, M., Ali, R. G., Paterson, A. D. & Yap, A. S. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr. Biol. 12, 379–382 (2002).
Yap, A. S. & Kovacs, E. M. Direct cadherin-activated cell signaling: a view from the plasma membrane. J. Cell Biol. 160, 11–16 (2003).
Braga, V. M. Cell–cell adhesion and signalling. Curr. Opin. Cell Biol. 14, 546–556 (2002).
Lampugnani, M. G. et al. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol. Biol. Cell 13, 1175–1189 (2002).
Noren, N. K., Niessen, C. M., Gumbiner, B. M. & Burridge, K. Cadherin engagement regulates Rho family GTPases. J. Biol. Chem. 276, 33305–33308 (2001).
Kovacs, E. M., Ali, R. G., McCormack, A. J. & Yap, A. S. E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J. Biol. Chem. 277, 6708–6718 (2002).
Hordijk, P. L. et al. Inhibition of invasion of epithelial cells by Tiam1–Rac signaling. Science 278, 1464–1466 (1997).
Sander, E. E., ten Klooster, J. P., van Delft, S., van der Kammen, R. A. & Collard, J. G. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 147, 1009–1022 (1999).
Malliri, A., van Es, S., Huveneers, S. & Collard, J. G. The Rac exchange factor Tiam1 is required for the establishment and maintenance of cadherin-based adhesions. J. Biol. Chem. 279, 30092–30098 (2004).
Mertens, A. E., Rygiel, T. P., Olivo, C., van der Kammen, R. & Collard, J. G. The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J. Cell Biol. 170, 1029–1037 (2005).
Yamazaki, D., Oikawa, T. & Takenawa, T. Rac–WAVE-mediated actin reorganization is required for organization and maintenance of cell–cell adhesion. J. Cell Sci. 120, 86–100 (2007).
Braga, V. M., Betson, M., Li, X. & Lamarche-Vane, N. Activation of the small GTPase Rac is sufficient to disrupt cadherin-dependent cell–cell adhesion in normal human keratinocytes. Mol. Biol. Cell 11, 3703–3721 (2000).
Vaezi, A., Bauer, C., Vasioukhin, V. & Fuchs, E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev. Cell 3, 367–381 (2002).
Zandy, N. L., Playford, M. & Pendergast, A. M. Abl tyrosine kinases regulate cell-cell adhesion through Rho GTPases. Proc. Natl Acad. Sci. USA 104, 17686–17691 (2007).
Dube, N. et al. The RapGEF PDZ-GEF2 is required for maturation of cell–cell junctions. Cell Signal. 20, 1608–1615 (2008).
Pannekoek, W. J., Kooistra, M. R., Zwartkruis, F. J. & Bos, J. L. Cell–cell junction formation: the role of Rap1 and Rap1 guanine nucleotide exchange factors. Biochim. Biophys. Acta 1788, 790–796 (2009).
Wildenberg, G. A. et al. p120-catenin and p190RhoGAP regulate cell–cell adhesion by coordinating antagonism between Rac and Rho. Cell 127, 1027–1039 (2006).
Lecuit, T. & Lenne, P. F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nature Rev. Mol. Cell Biol. 8, 633–644 (2007).
Harris, T. J., Sawyer, J. K. & Peifer, M. How the cytoskeleton helps build the embryonic body plan: models of morphogenesis from Drosophila. Curr. Top. Dev. Biol. 89, 55–85 (2009).
Chen, X. & Macara, I. G. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nature Cell Biol. 7, 262–269 (2005).
Delanoe-Ayari, H., Al Kurdi, R., Vallade, M., Gulino-Debrac, D. & Riveline, D. Membrane and acto-myosin tension promote clustering of adhesion proteins. Proc. Natl Acad. Sci. USA 101, 2229–2234 (2004).
Bard, L. et al. A molecular clutch between the actin flow and N-cadherin adhesions drives growth cone migration. J. Neurosci. 28, 5879–5890 (2008).
Fernandez-Gonzalez, R., Simoes Sde, M., Roper, J. C., Eaton, S. & Zallen, J. A. Myosin II dynamics are regulated by tension in intercalating cells. Dev. Cell 17, 736–743 (2009).
Shewan, A. M. et al. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol. Biol. Cell 16, 4531–4542 (2005).
Sahai, E. & Marshall, C. J. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nature Cell Biol. 4, 408–415 (2002).
Warner, S. J. & Longmore, G. D. Distinct functions for Rho1 in maintaining adherens junctions and apical tension in remodeling epithelia. J. Cell Biol. 185, 1111–1125 (2009).
Warner, S. J. & Longmore, G. D. Cdc42 antagonizes Rho1 activity at adherens junctions to limit epithelial cell apical tension. J. Cell Biol. 187, 119–133 (2009).
Zallen, J. A. Planar polarity and tissue morphogenesis. Cell 129, 1051–1063 (2007).
Zallen, J. A. & Wieschaus, E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev. Cell 6, 343–355 (2004).
Bertet, C., Sulak, L. & Lecuit, T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429, 667–671 (2004). Shows how localizing myosin activity to specific AJs can affect tissue morphogenesis.
Blankenship, J. T., Backovic, S. T., Sanny, J. S., Weitz, O. & Zallen, J. A. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev. Cell 11, 459–470 (2006).
Harris, T. J. & Peifer, M. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J. Cell Biol. 167, 135–147 (2004).
Cox, R. T., Kirkpatrick, C. & Peifer, M. Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophila embryogenesis. J. Cell Biol. 134, 133–148 (1996).
Barrett, K., Leptin, M. & Settleman, J. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell 91, 905–915 (1997).
Rogers, S. L., Wiedemann, U., Hacker, U., Turck, C. & Vale, R. D. Drosophila RhoGEF2 associates with microtubule plus ends in an EB1-dependent manner. Curr. Biol. 14, 1827–1833 (2004).
Costa, M., Wilson, E. T. & Wieschaus, E. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell 76, 1075–1089 (1994).
Kolsch, V., Seher, T., Fernandez-Ballester, G. J., Serrano, L. & Leptin, M. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science 315, 384–386 (2007).
Dawes-Hoang, R. E. et al. Folded gastrulation, cell shape change and the control of myosin localization. Development 132, 4165–4178 (2005).
Martin, A. C., Kaschube, M. & Wieschaus, E. F. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457, 495–499 (2009). Shows how myosin activity affecting all AJs across a tissue can cause apical constriction and tissue morphogenesis.
Stehbens, S. J., Akhmanova, A. & Yap, A. S. Microtubules and cadherins: a neglected partnership. Front. Biosci. 14, 3159–3167 (2009).
Stehbens, S. J. et al. Dynamic microtubules regulate the local concentration of E-cadherin at cell–cell contacts. J. Cell Sci. 119, 1801–1811 (2006).
Ligon, L. A. & Holzbaur, E. L. Microtubules tethered at epithelial cell junctions by dynein facilitate efficient junction assembly. Traffic 8, 808–819 (2007).
Bartolini, F. & Gundersen, G. G. Generation of noncentrosomal microtubule arrays. J. Cell Sci. 119, 4155–4163 (2006).
Waterman-Storer, C. M., Salmon, W. C. & Salmon, E. D. Feedback interactions between cell–cell adherens junctions and cytoskeletal dynamics in newt lung epithelial cells. Mol. Biol. Cell 11, 2471–2483 (2000).
Ligon, L. A., Karki, S., Tokito, M. & Holzbaur, E. L. Dynein binds to β-catenin and may tether microtubules at adherens junctions. Nature Cell Biol. 3, 913–917 (2001). Identifies a mechanism for linking microtubule plus ends to AJs.
Karki, S., Ligon, L. A., DeSantis, J., Tokito, M. & Holzbaur, E. L. PLAC-24 is a cytoplasmic dynein-binding protein that is recruited to sites of cell–cell contact. Mol. Biol. Cell 13, 1722–1734 (2002).
Chausovsky, A., Bershadsky, A. D. & Borisy, G. G. Cadherin-mediated regulation of microtubule dynamics. Nature Cell Biol. 2, 797–804 (2000).
Shtutman, M. et al. Signaling function of α-catenin in microtubule regulation. Cell Cycle 7, 2377–2383 (2008).
Mary, S. et al. Biogenesis of N-cadherin-dependent cell–cell contacts in living fibroblasts is a microtubule-dependent kinesin-driven mechanism. Mol. Biol. Cell 13, 285–301 (2002).
Tepass, U. & Hartenstein, V. The development of cellular junctions in the Drosophila embryo. Dev. Biol. 161, 563–596 (1994).
Harris, T. J. & Peifer, M. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J. Cell Biol. 170, 813–823 (2005).
Muller, H. A. & Wieschaus, E. Armadillo, Bazooka, and Stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J. Cell Biol. 134, 149–163 (1996).
Le Borgne, R., Bellaiche, Y. & Schweisguth, F. Drosophila E-cadherin regulates the orientation of asymmetric cell division in the sensory organ lineage. Curr. Biol. 12, 95–104 (2002).
Yamashita, Y. M., Jones, D. L. & Fuller, M. T. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 301, 1547–1550 (2003).
den Elzen, N., Buttery, C. V., Maddugoda, M. P., Ren, G. & Yap, A. S. Cadherin adhesion receptors orient the mitotic spindle during symmetric cell division in mammalian epithelia. Mol. Biol. Cell 20, 3740–3750 (2009).
Shaw, R. M. et al. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell 128, 547–560 (2007).
Nejsum, L. N. & Nelson, W. J. A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity. J. Cell Biol. 178, 323–335 (2007).
Dupin, I., Camand, E. & Etienne-Manneville, S. Classical cadherins control nucleus and centrosome position and cell polarity. J. Cell Biol. 185, 779–786 (2009).
Desai, R. A., Gao, L., Raghavan, S., Liu, W. F. & Chen, C. S. Cell polarity triggered by cell–cell adhesion via E-cadherin. J. Cell Sci. 122, 905–911 (2009). References 136–142 show how connections to AJs can affect the organization of microtubule networks.
Wirtz-Peitz, F. & Zallen, J. A. Junctional trafficking and epithelial morphogenesis. Curr. Opin. Genet. Dev. 19, 350–356 (2009).
Le, T. L., Yap, A. S. & Stow, J. L. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J. Cell Biol. 146, 219–232 (1999). Provides some of the first evidence for cadherin endocytosis and recycling.
de Beco, S., Gueudry, C., Amblard, F. & Coscoy, S. Endocytosis is required for E-cadherin redistribution at mature adherens junctions. Proc. Natl Acad. Sci. USA 106, 7010–7015 (2009).
Hong, S., Troyanovsky, R. B. & Troyanovsky, S. M. Spontaneous assembly and active disassembly balance adherens junction homeostasis. Proc. Natl Acad. Sci. USA 107, 3528–3533 (2010).
Schill, N. J. & Anderson, R. A. Out, in and back again: PtdIns(4,5)P2 regulates cadherin trafficking in epithelial morphogenesis. Biochem. J. 418, 247–260 (2009).
Troyanovsky, R. B., Sokolov, E. P. & Troyanovsky, S. M. Endocytosis of cadherin from intracellular junctions is the driving force for cadherin adhesive dimer disassembly. Mol. Biol. Cell 17, 3484–3493 (2006).
Gavard, J. & Gutkind, J. S. VEGF controls endothelial-cell permeability by promoting the β-arrestin-dependent endocytosis of VE-cadherin. Nature Cell Biol. 8, 1223–1234 (2006).
Chiasson, C. M., Wittich, K. B., Vincent, P. A., Faundez, V. & Kowalczyk, A. P. p120-catenin inhibits VE-cadherin internalization through a Rho-independent mechanism. Mol. Biol. Cell 20, 1970–1980 (2009).
Miyashita, Y. & Ozawa, M. Increased internalization of p120-uncoupled E-cadherin and a requirement for a dileucine motif in the cytoplasmic domain for endocytosis of the protein. J. Biol. Chem. 282, 11540–11548 (2007).
Classen, A. K., Anderson, K. I., Marois, E. & Eaton, S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev. Cell 9, 805–817 (2005). Shows how localizing cadherin recycling to specific cell–cell contacts can affect tissue morphogenesis.
Leibfried, A., Fricke, R., Morgan, M. J., Bogdan, S. & Bellaiche, Y. Drosophila Cip4 and WASp define a branch of the Cdc42–Par6–aPKC pathway regulating E-cadherin endocytosis. Curr. Biol. 18, 1639–1648 (2008).
Georgiou, M., Marinari, E., Burden, J. & Baum, B. Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr. Biol. 18, 1631–1638 (2008).
Fujita, Y. et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nature Cell Biol. 4, 222–231 (2002).
Xiao, K. et al. p120-catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol. Biol. Cell 16, 5141–5151 (2005).
Harris, K. P. & Tepass, U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J. Cell Biol. 183, 1129–1143 (2008).
D'Souza-Schorey, C. Disassembling adherens junctions: breaking up is hard to do. Trends Cell Biol. 15, 19–26 (2005).
Palacios, F., Schweitzer, J. K., Boshans, R. L. & D'Souza-Schorey, C. ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nature Cell Biol. 4, 929–936 (2002).
Kon, S., Tanabe, K., Watanabe, T., Sabe, H. & Satake, M. Clathrin dependent endocytosis of E-cadherin is regulated by the Arf196GAP isoform SMAP1. Exp. Cell Res. 314, 1415–1428 (2008).
Ikenouchi, J. & Umeda, M. FRMD4A regulates epithelial polarity by connecting Arf6 activation with the PAR complex. Proc. Natl Acad. Sci. USA 107, 748–753 (2010).
Ogata, S. et al. TGF-β signaling-mediated morphogenesis: modulation of cell adhesion via cadherin endocytosis. Genes Dev. 21, 1817–1831 (2007).
Shaye, D. D., Casanova, J. & Llimargas, M. Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea. Nature Cell Biol. 10, 964–970 (2008). References 162 and 163 provide examples of how global changes to cadherin endocytosis across a tissue can affect its morphogenesis.
Takai, Y., Ikeda, W., Ogita, H. & Rikitake, Y. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu. Rev. Cell Dev. Biol. 24, 309–342 (2008).
Tachibana, K. et al. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J. Cell Biol. 150, 1161–1176 (2000).
Pokutta, S., Drees, F., Takai, Y., Nelson, W. J. & Weis, W. I. Biochemical and structural definition of the l-afadin- and actin-binding sites of α-catenin. J. Biol. Chem. 277, 18868–18874 (2002).
Ikeda, W. et al. Afadin: A key molecule essential for structural organization of cell-cell junctions of polarized epithelia during embryogenesis. J. Cell Biol. 146, 1117–1132 (1999).
Larue, L., Ohsugi, M., Hirchenhain, J. & Kemler, R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc. Natl Acad. Sci. USA 91, 8263–8267 (1994).
Wei, S. Y. et al. Echinoid is a component of adherens junctions that cooperates with DE-Cadherin to mediate cell adhesion. Dev. Cell 8, 493–504 (2005).
Laplante, C. & Nilson, L. A. Differential expression of the adhesion molecule Echinoid drives epithelial morphogenesis in Drosophila. Development 133, 3255–3264 (2006).
Acknowledgements
The authors thank A. Koehler for the image in Figure 1. Work on adherens junctions in the authors' laboratories is supported by the Natural Sciences and Engineering Research Counsel of Canada (to T.J.C.H.), the Canadian Institutes of Health Research (to T.J.C.H. and U.T.) and the Canadian Cancer Society (to U.T.). T.J.C.H. is a Canada research chair in cell polarity and animal development.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Bilateran
-
An animal with a bilaterally symmetrical body plan, such as humans, fish and insects.
- Intermediate filament
-
A cytoskeletal filament that provides mechanical strength in higher eukaryotic cells.
- Puncta adherenta
-
Cadherin–catenin clusters found at cell–cell contacts, often during early stages of AJ assembly.
- Lamellipodium
-
A broad, flat protrusion at the leading edge of a moving cell that is enriched with a branched network of actin filaments.
- LIM domain
-
A repeat of about 60 amino acids, including Cys and His residues, that is thought to be involved in protein–protein interactions.
- Filopodium
-
A long, thin protrusion at the periphery of cells and growth cones. Filopodia are supported by F-actin bundles.
- Guanine nucleotide exchange factor (GEF)
-
A protein that facilitates the exchange of guanine diphosphate (GDP) for guanine triphosphate (GTP) in the nucleotide-binding pocket of a GTP-binding protein.
- Actomyosin
-
A complex of myosin and actin filaments that is responsible for contractility during a range of cellular movements in eukaryotic cells.
- GTPase-activating protein
-
(GAP). A protein that stimulates the intrinsic ability of a GTPase to hydrolyse GTP to GDP. Therefore, GAPs negatively regulate GTPases by converting them from an active (GTP-bound) to an inactive (GDP-bound) state.
- Microvillus
-
A small, finger-like projection that occurs on the exposed apical surfaces of epithelial cells. Microvilli are supported by F-actin bundles.
- Clathrin-coated pit
-
The initial site of invagination of a clathrin-coated vesicle.
- Notum
-
The dorsal side of the thorax of an adult insect.
- Epithelial-to-mesenchymal transition
-
The transformation of epithelial cells into mesenchymal cells with migratory and invasive properties.
Rights and permissions
About this article
Cite this article
Harris, T., Tepass, U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol 11, 502–514 (2010). https://doi.org/10.1038/nrm2927
Issue Date:
DOI: https://doi.org/10.1038/nrm2927
This article is cited by
-
PLK1 and its substrate MISP facilitate intrahepatic cholangiocarcinoma progression by promoting lymphatic invasion and impairing E-cadherin adherens junctions
Cancer Gene Therapy (2024)
-
Breaking through the basement membrane barrier to improve nanotherapeutic delivery to tumours
Nature Nanotechnology (2024)
-
Inhibition of miR-101-3p prevents human aortic valve interstitial cell calcification through regulation of CDH11/SOX9 expression
Molecular Medicine (2023)
-
Breaking barriers: exploring mechanisms behind opening the blood–brain barrier
Fluids and Barriers of the CNS (2023)
-
BCKDK regulates breast cancer cell adhesion and tumor metastasis by inhibiting TRIM21 ubiquitinate talin1
Cell Death & Disease (2023)