Key Points
-
PTEN (phosphatase and tensin homologue) is frequently disrupted in multiple sporadic tumours, and targeted by germline mutations in patients with cancer predisposition syndromes.
-
PTEN antagonizes the phosphoinositide 3-kinase (PI3K)–AKT–mammalian target of rapamycin (mTOR) pathway through its lipid phosphatase activity and governs a plethora of cellular processes including survival, proliferation, energy metabolism and cellular architecture.
-
The repertoire of PTEN functions has recently been expanded to include phosphatase-independent activities and crucial functions within the nucleus.
-
The molecular mechanisms regulating PTEN expression and function include transcriptional regulation, post-transcriptional regulation by microRNAs (miRNAs) and competitive endogenous RNA (ceRNAs), post-translational modifications and protein interactions.
-
The increasing knowledge of PTEN and pathologies that alter its function undoubtedly informs the rational design of novel cancer therapies.
Abstract
The importance of the physiological function of phosphatase and tensin homologue (PTEN) is illustrated by its frequent disruption in cancer. By suppressing the phosphoinositide 3-kinase (PI3K)–AKT–mammalian target of rapamycin (mTOR) pathway through its lipid phosphatase activity, PTEN governs a plethora of cellular processes including survival, proliferation, energy metabolism and cellular architecture. Consequently, mechanisms regulating PTEN expression and function, including transcriptional regulation, post-transcriptional regulation by non-coding RNAs, post-translational modifications and protein–protein interactions, are all altered in cancer. The repertoire of PTEN functions has recently been expanded to include phosphatase-independent activities and crucial functions within the nucleus. Our increasing knowledge of PTEN and pathologies in which its function is altered will undoubtedly inform the rational design of novel therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bigner, S. H., Mark, J., Mahaley, M. S. & Bigner, D. D. Patterns of the early, gross chromosomal changes in malignant human gliomas. Hereditas 101, 103–113 (1984).
Li, J. et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275, 1943–1947 (1997).
Steck, P. A. et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nature Genet. 15, 356–362 (1997).
Di Cristofano, A., Pesce, B., Cordon-Cardo, C. & Pandolfi, P. P. Pten is essential for embryonic development and tumour suppression. Nature Genet. 19, 348–355 (1998).
Suzuki, A. et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr. Biol. 8, 1169–1178 (1998).
Podsypanina, K. et al. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc. Natl Acad. Sci. USA 96, 1563–1568 (1999).
Di Cristofano, A., De Acetis, M., Koff, A., Cordon-Cardo, C. & Pandolfi, P. P. Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nature Genet. 27, 222–224 (2001).
Di Cristofano, A. et al. Impaired Fas response and autoimmunity in Pten+/− mice. Science 285, 2122–2125 (1999).
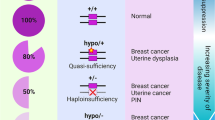
Alimonti, A. et al. Subtle variations in Pten dose determine cancer susceptibility. Nature Genet. 42, 454–458 (2010).
Berger, A. H., Knudson, A. G. & Pandolfi, P. P. A continuum model for tumour suppression. Nature 476, 163–169 (2011). References 9 and 10 demonstrate the crucial role of PTEN as a haploinsufficient tumour suppressor gene and propose a continuum model for tumour suppression.
Knudson, A. G. Mutation and cancer: statistical study of retinoblastoma. Proc. Natl Acad. Sci. USA 68, 820–823 (1971).
Maehama, T. & Dixon, J. E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273, 13375–13378 (1998).
Stambolic, V. et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95, 29–39 (1998).
Sun, H. et al. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5-trisphosphate and Akt/protein kinase B signaling pathway. Proc. Natl Acad. Sci. USA 96, 6199–6204 (1999).
Denu, J. M., Stuckey, J. A., Saper, M. A. & Dixon, J. E. Form and function in protein dephosphorylation. Cell 87, 361–364 (1996).
Myers, M. P. et al. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc. Natl Acad. Sci. USA 94, 9052–9057 (1997).
Lee, J. O. et al. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99, 323–334 (1999).
Raftopoulou, M., Etienne-Manneville, S., Self, A., Nicholls, S. & Hall, A. Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science 303, 1179–1181 (2004).
Manning, B. D. & Cantley, L. C. AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 (2007).
Zoncu, R., Efeyan, A. & Sabatini, D. M. mTOR: from growth signal integration to cancer, diabetes and ageing. Nature Rev. Mol. Cell Biol. 12, 21–35 (2011).
Guertin, D. A. & Sabatini, D. M. Defining the role of mTOR in cancer. Cancer Cell 12, 9–22 (2007).
Vander Haar, E., Lee, S. I., Bandhakavi, S., Griffin, T. J. & Kim, D. H. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nature Cell Biol. 9, 316–323 (2007).
Ma, X. M. & Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nature Rev. Mol. Cell Biol. 10, 307–318 (2009).
Dowling, R. J. et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 328, 1172–1176 (2010).
Hsieh, A. C. et al. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP–eIF4E. Cancer Cell 17, 249–261 (2010).
She, Q. B. et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell 18, 39–51 (2010). References 24, 25 and 26 describe a key role for 4EBP1-mediated translational control in the proliferation and survival of cancer cells.
Radimerski, T., Montagne, J., Hemmings-Mieszczak, M. & Thomas, G. Lethality of Drosophila lacking TSC tumor suppressor function rescued by reducing dS6K signaling. Genes Dev. 16, 2627–2632 (2002).
Zhang, H. et al. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J. Clin. Invest. 112, 1223–1233 (2003).
Harrington, L. S. et al. The TSC1–2 tumor suppressor controls insulin–PI3K signaling via regulation of IRS proteins. J. Cell Biol. 166, 213–223 (2004).
Um, S. H. et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431, 200–205 (2004).
Carracedo, A. et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J. Clin. Invest. 118, 3065–3074 (2008).
Kinkade, C. W. et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J. Clin. Invest. 118, 3051–3064 (2008). References 31 and 32 provide evidence that mTORC1 inhibitors result in increased activation of the RAS–MAPK pathway through a PI3K-dependent feedback loop.
Wan, X., Harkavy, B., Shen, N., Grohar, P. & Helman, L. J. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 26, 1932–1940 (2007).
Tamburini, J. et al. Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: rationale for therapeutic inhibition of both pathways. Blood 111, 379–382 (2008).
Hsu, P. P. et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 332, 1317–1322 (2011).
Yu, Y. et al. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science 332, 1322–1326 (2011). References 35 and 36 report GRB10 as an mTORC1 substrate and an effector of the mTORC1–PI3K feedback loop.
Manning, B. D. et al. Feedback inhibition of Akt signaling limits the growth of tumors lacking Tsc2. Genes Dev. 19, 1773–1778 (2005).
Nardella, C. et al. Aberrant Rheb-mediated mTORC1 activation and Pten haploinsufficiency are cooperative oncogenic events. Genes Dev. 22, 2172–2177 (2008).
Salmena, L., Carracedo, A. & Pandolfi, P. P. Tenets of PTEN tumor suppression. Cell 133, 403–414 (2008).
Warburg, O. On respiratory impairment in cancer cells. Science 124, 269–270 (1956).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009). This review comprehensively discusses the links between cell metabolism and cancer.
Eguez, L. et al. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2, 263–272 (2005).
Sundqvist, A. et al. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7). Cell Metab. 1, 379–391 (2005).
Li, X., Monks, B., Ge, Q. & Birnbaum, M. J. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1α transcription coactivator. Nature 447, 1012–1016 (2007).
Horie, Y. et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J. Clin. Invest. 113, 1774–1783 (2004).
Fang, M. et al. The ER UDPase ENTPD5 promotes protein N-glycosylation, the Warburg effect, and proliferation in the PTEN pathway. Cell 143, 711–724 (2010).
Kalaany, N. Y. & Sabatini, D. M. Tumours with PI3K activation are resistant to dietary restriction. Nature 458, 725–731 (2009).
Liliental, J. et al. Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr. Biol. 10, 401–404 (2000).
Tamura, M. et al. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science 280, 1614–1617 (1998).
Martin-Belmonte, F. et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128, 383–397 (2007).
Song, L. B. et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J. Clin. Invest. 119, 3626–3636 (2009).
Trimboli, A. J. et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature 461, 1084–1091 (2009).
Kalluri, R. & Zeisberg, M. Fibroblasts in cancer. Nature Rev. Cancer 6, 392–401 (2006).
Bronisz, A. et al. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nature Cell Biol. 14, 159–167 (2012). References 52 and 54 demonstrate a key role of PTEN in shaping the tumour microenvironment.
Kurose, K. et al. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nature Genet. 32, 355–357 (2002).
Hu, M. et al. Distinct epigenetic changes in the stromal cells of breast cancers. Nature Genet. 37, 899–905 (2005).
Lin, H. K. et al. Skp2 targeting suppresses tumorigenesis by Arf–p53-independent cellular senescence. Nature 464, 374–379 (2010).
Alimonti, A. et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J. Clin. Invest. 120, 681–693 (2010).
Chen, Z. et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436, 725–730 (2005). References 58 and 59 provide evidence that p53-mediated cellular senescence suppresses PTEN -deficient tumorigenesis.
Nardella, C. et al. Differential requirement of mTOR in postmitotic tissues and tumorigenesis. Sci. Signal. 2, ra2 (2009).
Chen, Z. et al. Differential p53-independent outcomes of p19(Arf) loss in oncogenesis. Sci. Signal. 2, ra44 (2009).
Palmero, I., Pantoja, C. & Serrano, M. p19ARF links the tumour suppressor p53 to Ras. Nature 395, 125–126 (1998).
Ohtani, N. et al. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature 409, 1067–1070 (2001).
Song, M. S. et al. Nuclear PTEN regulates the APC–CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell 144, 187–199 (2011).
Li, M. et al. The adaptor protein of the anaphase promoting complex Cdh1 is essential in maintaining replicative lifespan and in learning and memory. Nature Cell Biol. 10, 1083–1089 (2008).
Ding, Z. et al. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature 470, 269–273 (2011).
Nardella, C., Clohessy, J. G., Alimonti, A. & Pandolfi, P. P. Pro-senescence therapy for cancer treatment. Nature Rev. Cancer 11, 503–511 (2011).
Groszer, M. et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science 294, 2186–2189 (2001).
Bonaguidi, M. A. et al. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145, 1142–1155 (2011).
Zhang, J. et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature 441, 518–522 (2006).
Yilmaz, O. H. et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441, 475–482 (2006).
Ito, K., Bernardi, R. & Pandolfi, P. P. A novel signaling network as a critical rheostat for the biology and maintenance of the normal stem cell and the cancer-initiating cell. Curr. Opin. Genet. Dev. 19, 51–59 (2009).
Lee, J. Y. et al. mTOR activation induces tumor suppressors that inhibit leukemogenesis and deplete hematopoietic stem cells after Pten deletion. Cell Stem Cell 7, 593–605 (2010).
Guo, W. et al. Multi-genetic events collaboratively contribute to Pten-null leukaemia stem-cell formation. Nature 453, 529–533 (2008). References 70–74 demonstrate the crucial role of PTEN for cancer stem cell formation and haematopoietic stem cell homeostasis.
Lindsay, Y. et al. Localization of agonist-sensitive PtdIns(3,4,5)P3 reveals a nuclear pool that is insensitive to PTEN expression. J. Cell Sci. 119, 5160–5168 (2006).
Shen, W. H. et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128, 157–170 (2007). References 64 and 76 identify novel tumour suppressive functions of nuclear PTEN in its phosphatase-independent mode.
Puc, J. et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell 7, 193–204 (2005).
Mendes-Pereira, A. M. et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol. Med. 1, 315–322 (2009).
McEllin, B. et al. PTEN loss compromises homologous recombination repair in astrocytes: implications for glioblastoma therapy with temozolomide or poly(ADP-ribose) polymerase inhibitors. Cancer Res. 70, 5457–5464 (2010).
Dedes, K. J. et al. PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci. Transl. Med. 2, 53ra75 (2010).
Liu, X. S. et al. Polo-like kinase 1 facilitates loss of Pten tumor suppressor-induced prostate cancer formation. J. Biol. Chem. 286, 35795–35800 (2011).
Blanco-Aparicio, C., Renner, O., Leal, J. F. & Carnero, A. PTEN, more than the AKT pathway. Carcinogenesis 28, 1379–1386 (2007).
Kharas, M. G. et al. Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood 115, 1406–1415 (2010).
Vivanco, I. et al. Identification of the JNK signaling pathway as a functional target of the tumor suppressor PTEN. Cancer Cell 11, 555–569 (2007).
Okumura, K., Zhao, M., Depinho, R. A., Furnari, F. B. & Cavenee, W. K. Cellular transformation by the MSP58 oncogene is inhibited by its physical interaction with the PTEN tumor suppressor. Proc. Natl Acad. Sci. USA 102, 2703–2706 (2005).
Mounir, Z. et al. Tumor suppression by PTEN requires the activation of the PKR–eIF2α phosphorylation pathway. Sci. Signal. 2, ra85 (2009).
Gu, T. et al. CREB is a novel nuclear target of PTEN phosphatase. Cancer Res. 71, 2821–2825 (2011).
Zhang, S. et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nature Med. 17, 461–469 (2011).
Hollander, M. C., Blumenthal, G. M. & Dennis, P. A. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nature Rev. Cancer 11, 289–301 (2011).
Eng, C. PTEN: one gene, many syndromes. Hum. Mutat. 22, 183–198 (2003).
Trotman, L. C. et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell 128, 141–156 (2007).
Zhou, X. P. et al. Germline PTEN promoter mutations and deletions in Cowden/Bannayan-Riley-Ruvalcaba syndrome result in aberrant PTEN protein and dysregulation of the phosphoinositol-3-kinase/Akt pathway. Am. J. Hum. Genet. 73, 404–411 (2003).
Lu, J. et al. Stem cell factor SALL4 represses the transcriptions of PTEN and SALL1 through an epigenetic repressor complex. PLoS ONE 4, e5577 (2009).
Agrawal, S. & Eng, C. Differential expression of novel naturally occurring splice variants of PTEN and their functional consequences in Cowden syndrome and sporadic breast cancer. Hum. Mol. Genet. 15, 777–787 (2006).
Escriva, M. et al. Repression of PTEN phosphatase by Snail1 transcriptional factor during γ-radiation-induced apoptosis. Mol. Cell. Biol. 28, 1528–1540 (2008).
Lee, J. Y. et al. Id-1 activates Akt-mediated Wnt signaling and p27(Kip1) phosphorylation through PTEN inhibition. Oncogene 28, 824–831 (2009).
Stambolic, V. et al. Regulation of PTEN transcription by p53. Mol. Cell 8, 317–325 (2001).
Yoshimi, A. et al. Evi1 represses PTEN expression and activates PI3K/AKT/mTOR via interactions with polycomb proteins. Blood 117, 3617–3628 (2011).
Whelan, J. T., Forbes, S. L. & Bertrand, F. E. CBF-1 (RBP-Jκ) binds to the PTEN promoter and regulates PTEN gene expression. Cell Cycle 6, 80–84 (2007).
Hettinger, K. et al. c-Jun promotes cellular survival by suppression of PTEN. Cell Death Differ. 14, 218–229 (2007).
Xia, D. et al. Mitogen-activated protein kinase kinase-4 promotes cell survival by decreasing PTEN expression through an NF-κB-dependent pathway. J. Biol. Chem. 282, 3507–3519 (2007).
Patel, L. et al. Tumor suppressor and anti-inflammatory actions of PPARγ agonists are mediated via upregulation of PTEN. Curr. Biol. 11, 764–768 (2001).
Virolle, T. et al. The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nature Cell Biol. 3, 1124–1128 (2001).
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Xiao, C. et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17–92 expression in lymphocytes. Nature Immunol. 9, 405–414 (2008).
Olive, V. et al. miR-19 is a key oncogenic component of mir-17-92. Genes Dev. 23, 2839–2849 (2009).
Mavrakis, K. J. et al. Genome-wide RNA-mediated interference screen identifies miR-19 targets in Notch-induced T-cell acute lymphoblastic leukaemia. Nature Cell Biol. 12, 372–379 (2010). References 105, 106 and107 identify oncogenic miR-19 as PTEN-targeting for tumorigenesis.
Meng, F. et al. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 133, 647–658 (2007).
Ma, X. et al. Loss of the miR-21 allele elevates the expression of its target genes and reduces tumorigenesis. Proc. Natl Acad. Sci. USA 108, 10144–10149 (2011).
Mu, P. et al. Genetic dissection of the miR-17∼92 cluster of microRNAs in Myc-induced B-cell lymphomas. Genes Dev. 23, 2806–2811 (2009).
Poliseno, L. et al. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465, 1033–1038 (2010).
Salmena, L., Poliseno, L., Tay, Y., Kats, L. & Pandolfi, P. P. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 146, 353–358 (2011).
Karreth, F. A. et al. In vivo identification of tumor-suppressive PTEN ceRNAs in an oncogenic BRAF-induced mouse model of melanoma. Cell 147, 382–395 (2011).
Sumazin, P. et al. An extensive microRNA-mediated network of RNA–RNA interactions regulates established oncogenic pathways in glioblastoma. Cell 147, 370–381 (2011).
Tay, Y. et al. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell 147, 344–357 (2011). References 111–115 provide evidence that RNA transcripts communicate through a new 'language' mediated by miRNA-binding sites.
Rabinovsky, R. et al. p85 associates with unphosphorylated PTEN and the PTEN-associated complex. Mol. Cell. Biol. 29, 5377–5388 (2009).
Vazquez, F. et al. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J. Biol. Chem. 276, 48627–48630 (2001).
Liang, K. et al. Recombinant human erythropoietin antagonizes trastuzumab treatment of breast cancer cells via Jak2-mediated Src activation and PTEN inactivation. Cancer Cell 18, 423–435 (2010).
Al-Khouri, A. M., Ma, Y., Togo, S. H., Williams, S. & Mustelin, T. Cooperative phosphorylation of the tumor suppressor phosphatase and tensin homologue (PTEN) by casein kinases and glycogen synthase kinase 3β. J. Biol. Chem. 280, 35195–35202 (2005).
Xu, D., Yao, Y., Jiang, X., Lu, L. & Dai, W. Regulation of PTEN stability and activity by Plk3. J. Biol. Chem. 285, 39935–39942 (2010).
Li, Z. et al. Regulation of PTEN by Rho small GTPases. Nature Cell Biol. 7, 399–404 (2005).
Yim, E. K. et al. Rak functions as a tumor suppressor by regulating PTEN protein stability and function. Cancer Cell 15, 304–314 (2009).
Wang, X. et al. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell 128, 129–139 (2007).
Okumura, K. et al. PCAF modulates PTEN activity. J. Biol. Chem. 281, 26562–26568 (2006).
Chae, H. D. & Broxmeyer, H. E. SIRT1 deficiency downregulates PTEN/JNK/FOXO1 pathway to block reactive oxygen species-induced apoptosis in mouse embryonic stem cells. Stem Cells Dev. 20, 1277–1285 (2011).
Lee, S. R. et al. Reversible inactivation of the tumor suppressor PTEN by H2O2 . J. Biol. Chem. 277, 20336–20342 (2002).
Silva, A. et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J. Clin. Invest. 118, 3762–3774 (2008).
Hui, S. T. et al. Txnip balances metabolic and growth signaling via PTEN disulfide reduction. Proc. Natl Acad. Sci. USA 105, 3921–3926 (2008).
Cao, J. et al. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 28, 1505–1517 (2009).
Drinjakovic, J. et al. E3 ligase Nedd4 promotes axon branching by downregulating PTEN. Neuron 65, 341–357 (2010).
Fouladkou, F. et al. The ubiquitin ligase Nedd4-1 is dispensable for the regulation of PTEN stability and localization. Proc. Natl Acad. Sci. USA 105, 8585–8590 (2008).
Van Themsche, C., Leblanc, V., Parent, S. & Asselin, E. X-linked inhibitor of apoptosis protein (XIAP) regulates PTEN ubiquitination, content, and compartmentalization. J. Biol. Chem. 284, 20462–20466 (2009).
Maddika, S. et al. WWP2 is an E3 ubiquitin ligase for PTEN. Nature Cell Biol. 13, 728–733 (2011).
Gunaratne, J. et al. Protein interactions of phosphatase and tensin homologue (PTEN) and its cancer-associated G20E mutant compared by using stable isotope labeling by amino acids in cell culture-based parallel affinity purification. J. Biol. Chem. 286, 18093–18103 (2011).
Song, M. S. et al. The deubiquitinylation and localization of PTEN are regulated by a HAUSP–PML network. Nature 455, 813–817 (2008). References 91, 123 and 135 provide evidence that PTEN is modified by an ubiquitylation–deubiquitylation network.
Takahashi, Y., Morales, F. C., Kreimann, E. L. & Georgescu, M. M. PTEN tumor suppressor associates with NHERF proteins to attenuate PDGF receptor signaling. EMBO J. 25, 910–920 (2006).
Molina, J. R. et al. PTEN, NHERF1 and PHLPP form a tumor suppressor network that is disabled in glioblastoma. Oncogene 31, 1264–1274 (2012).
Lima-Fernandes, E. et al. Distinct functional outputs of PTEN signalling are controlled by dynamic association with β-arrestins. EMBO J. 30, 2557–2568 (2011).
Wu, X. et al. Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc. Natl Acad. Sci. USA 97, 4233–4238 (2000).
van Diepen, M. T. et al. MyosinV controls PTEN function and neuronal cell size. Nature Cell Biol. 11, 1191–1196 (2009).
Cotter, L. et al. Dlg1–PTEN interaction regulates myelin thickness to prevent damaging peripheral nerve overmyelination. Science 328, 1415–1418 (2010).
Chagpar, R. B. et al. Direct positive regulation of PTEN by the p85 subunit of phosphatidylinositol 3-kinase. Proc. Natl Acad. Sci. USA 107, 5471–5476 (2010).
Taniguchi, C. M. et al. The phosphoinositide 3-kinase regulatory subunit p85α can exert tumor suppressor properties through negative regulation of growth factor signaling. Cancer Res. 70, 5305–5315 (2010).
Kim, R. H. et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell 7, 263–273 (2005).
Kim, Y. C., Kitaura, H., Taira, T., Iguchi-Ariga, S. M. & Ariga, H. Oxidation of DJ-1-dependent cell transformation through direct binding of DJ-1 to PTEN. Int. J. Oncol. 35, 1331–1341 (2009).
Fine, B. et al. Activation of the PI3K pathway in cancer through inhibition of PTEN by exchange factor P-REX2a. Science 325, 1261–1265 (2009).
He, L., Ingram, A., Rybak, A. P. & Tang, D. Shank-interacting protein-like 1 promotes tumorigenesis via PTEN inhibition in human tumor cells. J. Clin. Invest. 120, 2094–2108 (2010).
He, L. et al. α-mannosidase 2C1 attenuates PTEN function in prostate cancer cells. Nature Commun. 2, 307 (2011).
Engelman, J. A., Luo, J. & Cantley, L. C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nature Rev. Genet. 7, 606–619 (2006).
Vanhaesebroeck, B., Guillermet-Guibert, J., Graupera, M. & Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nature Rev. Mol. Cell Biol. 11, 329–341 (2010).
Juhasz, G. et al. The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J. Cell Biol. 181, 655–666 (2008).
Scheid, M. P. & Woodgett, J. R. PKB/AKT: functional insights from genetic models. Nature Rev. Mol. Cell Biol. 2, 760–768 (2001).
Liaw, D. et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nature Genet. 16, 64–67 (1997).
Backman, S. A. et al. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nature Genet. 29, 396–403 (2001).
Kwon, C. H. et al. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nature Genet. 29, 404–411 (2001).
Meng, F. et al. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology 130, 2113–2129 (2006).
Carver, B. S. et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nature Genet. 41, 619–624 (2009).
King, J. C. et al. Cooperativity of TMPRSS2–ERG with PI3-kinase pathway activation in prostate oncogenesis. Nature Genet. 41, 524–526 (2009). References 157 and 158 provide evidence that ERG (v-ets erythroblastosis virus E26 oncogene homologue) cooperates with PTEN to promote cancer progression.
Okahara, F., Ikawa, H., Kanaho, Y. & Maehama, T. Regulation of PTEN phosphorylation and stability by a tumor suppressor candidate protein. J. Biol. Chem. 279, 45300–45303 (2004).
Acknowledgements
The authors are grateful to all members of the Pandolfi laboratory for insightful comments and discussion. The authors also thank H. M. Kim for providing the modified PTEN structures. L.S. was supported by fellowships from the Canadian Institutes of Health Research and the Human Frontier Science Program. This work was supported by US National Institutes of Health (NIH) grant R01 CA-82328-09 awarded to P.P.P.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Related links
Related links
FURTHER INFORMATION
Pier Paolo Pandolfi's homepage
The Sanger Institute Catalogue of Somatic Mutations in Cancer (COSMIC)
SUPPLEMENTARY INFORMATION
Glossary
- Cowden disease
-
An autosomal dominant multiple hamartoma syndrome that results most commonly (in 80% of cases) from a mutation in the phosphatase and tensin homologue (PTEN) gene. The disease was named after the family in which it was first reported. Although the tumours are largely benign, people with Cowden syndrome have an increased risk of developing several types of cancer, including cancers of the breast, thyroid and uterus.
- Haploinsufficient tumour suppressor
-
A tumour suppressor gene is haploinsufficient when loss of one functional copy in a diploid organism compromises tumour suppression, because the remaining copy of the gene is incapable of providing sufficient protein for tumour suppressive function.
- Two-hit model
-
Mutation or deletion of one allele induces cancer susceptibility (the first hit) and mutation or loss of the other allele induces cancer (the second hit).
- C2 domains
-
Protein structural domains involved in targeting proteins to cell membranes. They are composed of eight β-strands that coordinate calcium ions, which bind in a cavity formed by the first and final loops of the domain on the membrane binding face.
- PDZ domain
-
(Postsynaptic-density protein of 95 kDa, discs large, zona occludens-1). A protein-interaction domain that often occurs in scaffolding proteins and is named after the founding members of this protein family.
- Pleckstrin homology domains
-
(PH domains). Protein structural domains composed of two perpendicular antiparallel β-sheets followed by a carboxy-terminal α-helix. They are capable of binding phosphatidylinositol lipids in cell membranes and thus targeting proteins to the membranes.
- Metabolic reprogramming
-
Reprogramming of cellular metabolism allows quiescent cells to proliferate. Proliferating cells often take up nutrients in excess of bioenergetic needs (for example, ATP) and shunt metabolites into pathways that support a platform for biosynthesis (for example, amino acids, lipids and nucleic acids).
- Warburg effect
-
A phenomenon first described by Otto Warburg in which rapidly proliferating tumour cells consume glucose at a higher rate than normal cells and secrete most of the glucose-derived carbon as lactate rather than oxidizing it completely.
- Pro-senescence therapy
-
Novel therapeutic approach to treat cancers by inducing cellular senescence — a robust physiological antitumour response that is engaged by tissues to counteract oncogenic insults. Senescent cells can be cleared through an innate immune response.
- PTEN hamartoma tumour syndromes
-
(PHTSs). Patients with PHTS harbour phosphatase and tensin homologue (PTEN) mutations and develop non-malignant tumours. In PHTSs the proliferation of normal cellular elements is accompanied by disordered architecture. PHTSs include Cowden syndrome, Bannayan–Riley–Ruvalcaba syndrome (BRRS), Proteus syndrome (PS), Proteus-like syndrome (PSL) and autism spectrum disorder with macrocephaly.
- miR-17∼92 cluster
-
The miR-17∼92 cluster is a polycistron that generates six different miRNAs (miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1 and miR-92a) from one transcript.
- ceRNA hypothesis
-
A unifying hypothesis introducing a new level of post-transcriptional regulation. According to this hypothesis, different RNA transcripts are competing for binding of microRNAs (miRNAs) to their miRNA-binding sites ('miRNA response elements' or 'MREs').
- Acute promyelocytic leukaemia
-
(APL). A subtype of acute myeloid leukaemia that stems from a t(15;17)(q24;q21) translocation. APL is characterized by an abnormal accumulation of immature granulocytes called promyelocytes.
Rights and permissions
About this article
Cite this article
Song, M., Salmena, L. & Pandolfi, P. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol 13, 283–296 (2012). https://doi.org/10.1038/nrm3330
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3330
This article is cited by
-
Deciphering the role of neddylation in tumor microenvironment modulation: common outcome of multiple signaling pathways
Biomarker Research (2024)
-
Sirt6 ablation in the liver causes fatty liver that increases cancer risky by upregulating Serpina12
EMBO Reports (2024)
-
Recent advances in the roles of exosomal microRNAs (exomiRs) in hematologic neoplasms: pathogenesis, diagnosis, and treatment
Cell Communication and Signaling (2023)
-
circACTR2 attenuates gemcitabine chemoresiatance in pancreatic cancer through PTEN mediated PI3K/AKT signaling pathway
Biology Direct (2023)
-
Elevated ZNF704 expression is associated with poor prognosis of uveal melanoma and promotes cancer cell growth by regulating AKT/mTOR signaling
Biomarker Research (2023)