Abstract
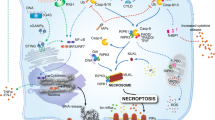
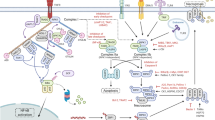
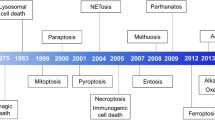
Cell death research was revitalized by the understanding that necrosis can occur in a highly regulated and genetically controlled manner. Although RIPK1 (receptor-interacting protein kinase 1)- and RIPK3–MLKL (mixed lineage kinase domain-like)-mediated necroptosis is the most understood form of regulated necrosis, other examples of this process are emerging, including cell death mechanisms known as parthanatos, oxytosis, ferroptosis, NETosis, pyronecrosis and pyroptosis. Elucidating how these pathways of regulated necrosis are interconnected at the molecular level should enable this process to be therapeutically targeted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vogt, C. Untersuchungen über die Entwicklungsgeschichte der Geburtshelferkröte (Alytes obstetricans). (Solothurn: Jent und Gassmann, 1842).
Lockshin, R. A. & Williams, C. M. Programmed cell death. II. Endocrine potentiation of the breakdown of the intersegmental muscles of silkmoths. J. Insect. Physiol. 10, 643–649 (1964).
Schweichel, J. U. & Merker, H. J. The morphology of various types of cell death in prenatal tissues. Teratology 7, 253–266 (1973).
Ellis, H. M. & Horvitz, H. R. Genetic control of programmed cell death in the nematode C. elegans. Cell 44, 817–829 (1986).
Crawford, E. D. & Wells, J. A. Caspase substrates and cellular remodeling. Annu. Rev. Biochem. 80, 1055–1087 (2011).
Suzanne, M. & Steller, H. Shaping organisms with apoptosis. Cell Death Differ. 20, 669–675 (2013).
Kaczmarek, A., Vandenabeele, P. & Krysko, D. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 38, 209–223 (2013).
Taylor, R., Cullen, S. & Martin, S. Apoptosis: controlled demolition at the cellular level. Nature Rev. Mol. Cell Biol. 9, 231–241 (2008).
Holler, N. et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nature Immunol. 1, 489–495 (2000).
Sun, L. et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012).
Vandenabeele, P., Galluzzi, L., Vanden Berghe, T. & Kroemer, G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nature Rev. Mol. Cell Biol. 11, 700–714 (2010).
Zhang, D. W. et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336 (2009).
Cho, Y. S. et al. Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
Feng, S. et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 19, 2056–2067 (2007).
He, S. et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 137, 1100–1111 (2009).
Degterev, A. et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nature Chem. Biol. 4, 313–321 (2008).
Teng, X. et al. Structure–activity relationship study of novel necroptosis inhibitors. Bioorg. Med. Chem. Lett. 15, 5039–5044 (2005).
Degterev, A. et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nature Chem. Biol. 1, 112–119 (2005).
Laster, S., Wood, J. & Gooding, L. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J. Immunol. 141, 2629–2634 (1988).
Grooten, J., Goossens, V., Vanhaesebroeck, B. & Fiers, W. Cell membrane permeabilization and cellular collapse, followed by loss of dehydrogenase activity: early events in tumour necrosis factor-induced cytotoxicity. Cytokine 5, 546–555 (1993).
Vercammen, D. et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 187, 1477–1485 (1998).
Kalai, M. et al. Tipping the balance between necrosis and apoptosis in human and murine cells treated with interferon and dsRNA. Cell Death Differ. 9, 981–994 (2002).
Galluzzi, L. et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 19, 107–120 (2012).
Wertz, I. E. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 430, 694–699 (2004).
Wilson, N. S., Dixit, V. & Ashkenazi, A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nature Immunol. 10, 348–355 (2009).
Li, J. et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150, 339–350 (2012).
Moquin, D., McQuade, T. & Chan, F. CYLD deubiquitinates RIP1 in the TNFα-induced necrosome to facilitate kinase activation and programmed necrosis. PloS ONE 8, e76841 (2013).
Chen, W. et al. Diverse sequence determinants control human and mouse receptor interacting protein 3 (RIP3) and mixed lineage kinase domain-like (MLKL) interaction in necroptotic signaling. J. Biol. Chem. 288, 16247–16261 (2013).
McQuade, T., Cho, Y. & Chan, F. positive and negative phosphorylation regulates RIP1 and RIP3-induced programmed necrosis. Biochem. J. 456, 409–415 (2013).
Xie, T. et al. Structural insights into RIP3-mediated necroptotic signaling. Cell Rep. 5, 70–78 (2013).
Zhao, J. et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc. Natl Acad. Sci. USA 109, 5322–5327 (2012).
Shulga, N. & Pastorino, J. GRIM-19-mediated translocation of STAT3 to mitochondria is necessary for TNF-induced necroptosis. J. Cell Sci. 125, 2995–3003 (2012).
Schulze-Osthoff, K. et al. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J. Biol. Chem. 267, 5317–5323 (1992).
Tait, Stephen, W. G. et al. Widespread mitochondrial depletion via mitophagy does not compromise necroptosis. Cell Rep. 5, 878–885 (2013).
Murphy, J. et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39, 443–453 (2013).
Wu, J. et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 23, 994–1006 (2013).
Du, C., Fang, M., Li, Y., Li, L. & Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102, 33–42 (2000).
Varfolomeev, E. et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kB activation, and TNF-dependent apoptosis. Cell 131, 669–681 (2007).
Vince, J. E. et al. IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell 131, 682–693 (2007).
Bertrand, M. J. et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 (2008).
Yang, Q.-H. & Du, C. Smac/DIABLO selectively reduces the levels of c-IAP1 and c-IAP2 but not that of XIAP and livin in HeLa cells. J. Biol. Chem. 279, 16963–16970 (2004).
Varfolomeev, E. et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor α (TNFα)-induced NF-κB activation. J. Biol. Chem. 283, 24295–24299 (2008).
Mahoney, D. J. et al. Both cIAP1 and cIAP2 regulate TNFα-mediated NF-κB activation. Proc. Natl Acad. Sci. USA 105, 11778–11783 (2008).
Zarnegar, B. J. et al. Noncanonical NF-κB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nature Immunol. 9, 1371–1378 (2008).
Vanlangenakker, N., Vanden Berghe, T., Krysko, D. V., Festjens, N. & Vandenabeele, P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr. Mol. Med. 8, 207–220 (2008).
Galluzzi, L. et al. Programmed necrosis from molecules to health and disease. Int. Rev. Cell. Mol. Biol. 289, 1–35 (2011).
Moriwaki, K. & Chan, F. RIP3: a molecular switch for necrosis and inflammation. Genes Dev. 27, 1640–1649 (2013).
Zhou, Z., Han, V. & Han, J. New components of the necroptotic pathway. Protein Cell 3, 811–817 (2012).
Newton, K., Sun, X. & Dixit, V. M. Kinase RIP3 is dispensable for normal NF-κBs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol. Cell Biol. 24, 1464–1469 (2004).
Kaiser, W., Upton, J. & Mocarski, E. Viral modulation of programmed necrosis. Curr. Opin. Virol. 3, 296–306 (2013).
Mack, C., Sickmann, A., Lembo, D. & Brune, W. Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein. Proc. Natl Acad. Sci. USA 105, 3094–3099 (2008).
Skaletskaya, A. et al. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl Acad. Sci. USA 98, 7829–7834 (2001).
Wang, L., Du, F. & Wang, X. TNF-α induces two distinct caspase-8 activation pathways. Cell 133, 693–703 (2008).
Takahashi, N. et al. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 3, e437 (2012).
Degterev, A., Maki, J. & Yuan, J. Activity and specificity of necrostatin-1, small-molecule inhibitor of RIP1 kinase. Cell Death Differ. 20, 366 (2013).
Zheng, W., Degterev, A., Hsu, E., Yuan, J. & Yuan, C. Structure–activity relationship study of a novel necroptosis inhibitor, necrostatin-7. Bioorg. Med. Chem. Lett. 18, 4932–4935 (2008).
Wu, Z., Li, Y., Cai, Y., Yuan, J. & Yuan, C. A novel necroptosis inhibitor-necrostatin-21 and its SAR study. Bioorg. Med. Chem. Lett. 23, 4903–4906 (2013).
Kaiser, W. et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3 and MLKL. J. Biol. Chem. 288, 31268–31279 (2013).
Morgan, M., Kim, Y.-S. & Liu, Z.-G. Membrane-bound Fas ligand requires RIP1 for efficient activation of caspase-8 within the death-inducing signaling complex. J. Immunol. 183, 3278–3284 (2009).
Kang, T.-B., Yang, S.-H., Toth, B., Kovalenko, A. & Wallach, D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 38, 27–40 (2013).
Lukens, J. et al. RIP1-driven autoinflammation targets IL-1α independently of inflammasomes and RIP3. Nature 498, 224–227 (2013).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell http://dx.doi.org/10.1016/j.cell.2013.12.010 (2014).
Kurz, T., Gustafsson, B. & Brunk, U. T. Intralysosomal iron chelation protects against oxidative stress-induced cellular damage. FEBS J. 273, 3106–3117 (2006).
Vanden Berghe, T. et al. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 17, 922–930 (2010).
Tan, S., Schubert, D. & Maher, P. Oxytosis: novel form of programmed cell death. Curr. Top. Med. Chem. 1, 497–506 (2001).
Pérez-De La Cruz, V., Carrillo-Mora, P. & Santamaría, A. Quinolinic acid, an endogenous molecule combining excitotoxicity, oxidative stress and other toxic mechanisms. Int. J. Tryptophan Res. 5, 1–8 (2012).
Henke, N. et al. The plasma membrane channel ORAI1 mediates detrimental calcium influx caused by endogenous oxidative stress. Cell Death Dis. 4, e470 (2013).
Yamashima, T. Ca2+-dependent proteases in ischemic neuronal death: a conserved 'calpain–cathepsin cascade' from nematodes to primates. Cell Calcium 36, 285–293 (2004).
Syntichaki, P., Xu, K., Driscoll, M. & Tavernarakis, N. Specific aspartyl and calpain proteases are required for neurodegeneration in C. elegans. Nature 419, 939–944 (2002).
Seiler, A. et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 8, 237–248 (2008).
Kleikers, P. et al. NADPH oxidases as a source of oxidative stress and molecular target in ischemia/reperfusion injury. J. Mol. Med. 90, 1391–1406 (2012).
Valencia, A. et al. Elevated NADPH oxidase activity contributes to oxidative stress and cell death in Huntington's disease. Hum. Mol. Genet. 22, 1112–1131 (2013).
Song, S. X. et al. Attenuation of brain edema and spatial learning de fi cits by the inhibition of NADPH oxidase activity using apocynin following diffuse traumatic brain injury in rats. Mol. Med. Rep. 7, 327–331 (2013).
Zhang, M., Perino, A., Ghigo, A., Hirsch, E. & Shah, A. NADPH oxidases in heart failure: poachers or gamekeepers? Antioxid. Redox Signal. 18, 1024–1041 (2013).
Yazdanpanah, B. et al. Riboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature 460, 1159–1163 (2009).
Gabelloni, M. L. et al. NADPH oxidase derived reactive oxygen species are involved in human neutrophil IL-1β secretion but not in inflammasome activation. Eur. J. Immunol. 43, 3324–3335 (2013).
Sokolovska, A. et al. Activation of caspase-1 by the NLRP3 inflammasome regulates the NADPH oxidase NOX2 to control phagosome function. Nature Immunol. 14, 543–553 (2013).
Remijsen, Q. et al. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 18, 581–588 (2011).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Remijsen, Q. et al. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 21, 290–304 (2011).
Wartha, F. & Henriques-Normark, B. ETosis: a novel cell death pathway. Sci. Signal. 1, pe25 (2008).
Yipp, B. et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nature Med. 18, 1386–1393 (2012).
van den Berg, J. et al. Chronic granulomatous disease: the European experience. PloS ONE 4, e5234 (2009).
Elrod, J. & Molkentin, J. Physiologic functions of cyclophilin d and the mitochondrial permeability transition pore. Circ. J. 77, 1111–1122 (2013).
Javadov, S. & Kuznetsov, A. Mitochondrial permeability transition and cell death: the role of cyclophilin D. Front. Physiol. 4, 76 (2013).
Baines, C. P. et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434, 658–662 (2005).
Clarke, S., McStay, G. & Halestrap, A. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J. Biol. Chem. 277, 34793–34799 (2002).
Schinzel, A. C. et al. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl Acad. Sci. USA 102, 12005–12010 (2005).
Bonora, M. et al. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle 12, 674–683 (2013).
Giorgio, V. et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl Acad. Sci. USA 110, 5887–5892 (2013).
Nakagawa, T. et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434, 652–658 (2005).
Basso, E. et al. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J. Biol. Chem. 280, 18558–18561 (2005).
Kokoszka, J. E. et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427, 461–465 (2004).
Devalaraja-Narashimha, K., Diener, A. & Padanilam, B. Cyclophilin D gene ablation protects mice from ischemic renal injury. Am. J. Physiol. Renal Physiol. 297, 59 (2009).
Piot, C. et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. New Engl. J. Med. 359, 473–481 (2008).
Vaseva, A. et al. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 149, 1536–1548 (2012).
Baumann, K. Cell death: multitasking p53 promotes necrosis. Nature reviews. Mol. Cell Biol. 13, 480–481 (2012).
Karch, J. & Molkentin, J. Is p53 the long-sought molecular trigger for cyclophilin D-regulated mitochondrial permeability transition pore formation and necrosis? Circul. Res. 111, 1258–1260 (2012).
Linkermann, A. et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc. Natl Acad. Sci. USA 110, 12024–12029 (2013).
Gibson, B. & Kraus, W. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nature Rev. Mol. Cell Biol. 13, 411–424 (2012).
Lonskaya, I. et al. Regulation of poly(ADP-ribose) polymerase-1 by DNA structure-specific binding. J. Biol. Chem. 280, 17076–17083 (2005).
Bürkle, A. & Virág, L. Poly(ADP-ribose): PARadigms and PARadoxes. Mol. Aspects Med. 34, 1046–1065 (2013).
Andrabi, S., Dawson, T. & Dawson, V. Mitochondrial and nuclear cross talk in cell death: parthanatos. Ann. NY Acad. Sci. 1147, 233–241 (2008).
Los, M. et al. Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol. Biol. Cell 13, 978–988 (2002).
Simbulan-Rosenthal, C. M. et al. Inhibition of poly(ADP-ribose) polymerase activity is insufficient to induce tetraploidy. Nucleic Acids Res. 29, 841–849 (2001).
Virag, L., Robaszkiewicz, A., Vargas, J. M. & Javier Oliver, F. Poly(ADP-ribose) signaling in cell death. Mol. Aspects Med. 34, 1153–1167 (2013).
Jagtap, P. & Szabó, C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nature Rev. Drug Discov. 4, 421–440 (2005).
Curtin, N. & Szabo, C. Therapeutic applications of PARP inhibitors: anticancer therapy and beyond. Mol. Aspects Med. 34, 1217–56 (2013).
Jouan-Lanhouet, S. et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 19, 2003–2014 (2012).
Sosna, J. et al. TNF-induced necroptosis and PARP-1-mediated necrosis represent distinct routes to programmed necrotic cell death. Cell Mol. Life Sci. http://dx.doi.org/10.1007/s00018-013-1381-6 (2013)
Xu, X. et al. The role of PARP activation in glutamate-induced necroptosis in HT-22 cells. Brain Res. 1343, 206–212 (2010).
Cookson, B. & Brennan, M. Pro-inflammatory programmed cell death. Trends Microbiol. 9, 113–114 (2001).
von Moltke, J., Ayres, J. S., Kofoed, E. M., Chavarria-Smith, J. & Vance, R. E. Recognition of bacteria by inflammasomes. Annu. Rev. Immunol. 31, 73–106 (2013).
Fink, S. & Cookson, B. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 8, 1812–1825 (2006).
Brennan, M. & Cookson, B. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 38, 31–40 (2000).
Miao, E. et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nature Immunol. 11, 1136–1142 (2010).
Sauer, J.-D. et al. Listeria monocytogenes engineered to activate the Nlrc4 inflammasome are severely attenuated and are poor inducers of protective immunity. Proc. Natl Acad. Sci. USA 108, 12419–12424 (2011).
Case, C., Shin, S. & Roy, C. Asc and Ipaf inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infection Immun. 77, 1981–1991 (2009).
Zamboni, D. et al. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nature Immunol. 7, 318–325 (2006).
Derré, I. & Isberg, R. Macrophages from mice with the restrictive Lgn1 allele exhibit multifactorial resistance to Legionella pneumophila. Infection Immun. 72, 6221–6229 (2004).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011).
Case, C. et al. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc. Natl Acad. Sci. USA 110, 1851–1856 (2013).
Aachoui, Y. et al. Caspase-11 protects against bacteria that escape the vacuole. Science 339, 975–978 (2013).
Hagar, J., Powell, D., Aachoui, Y., Ernst, R. & Miao, E. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013).
Rathinam, V. et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150, 606–619 (2012).
Willingham, S. et al. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe 2, 147–159 (2007).
Duncan, J. et al. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J. Immunol. 182, 6460–6469 (2009).
Zhao, Y., Khaminets, A., Hunn, J. & Howard, J. Disruption of the Toxoplasma gondii parasitophorous vacuole by IFNγ-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death. PLoS Pathog. 5, e1000288 (2009).
Averette, K. et al. Anthrax lethal toxin induced lysosomal membrane permeabilization and cytosolic cathepsin release is Nlrp1b/Nalp1b-dependent. PloS ONE 4, e7913 (2009).
Holzinger, D. et al. Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J. Leukocyte Biol. 92, 1069–1081 (2012).
Oka, S.-I., Hsu, C.-P. & Sadoshima, J. Regulation of cell survival and death by pyridine nucleotides. Circul. Res. 111, 611–627 (2012).
Kristian, T., Balan, I., Schuh, R. & Onken, M. Mitochondrial dysfunction and nicotinamide dinucleotide catabolism as mechanisms of cell death and promising targets for neuroprotection. J. Neurosci. Res. 89, 1946–1955 (2011).
Belenky, P., Bogan, K. & Brenner, C. NAD+ metabolism in health and disease. Trends Biochem. Sci. 32, 12–19 (2007).
Sattler, R. & Tymianski, M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol. Neurobiol. 24, 107–129 (2001).
Yoshida, M. et al. Primate neurons show different vulnerability to transient ischemia and response to cathepsin inhibition. Acta Neuropathol. 104, 267–272 (2002).
Bano, D. et al. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell 120, 275–285 (2005).
Orabi, A. et al. Dantrolene mitigates caerulein-induced pancreatitis in vivo in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G196–G204 (2010).
Staats, K. et al. Dantrolene is neuroprotective in vitro, but does not affect survival in SOD1G93A mice. Neuroscience 220, 26–31 (2012).
Mattson, M., Zhu, H., Yu, J. & Kindy, M. Presenilin-1 mutation increases neuronal vulnerability to focal ischemia in vivo and to hypoxia and glucose deprivation in cell culture: involvement of perturbed calcium homeostasis. J. Neurosci. 20, 1358–1364 (2000).
Chen, X. et al. Dantrolene is neuroprotective in Huntington's disease transgenic mouse model. Mol. Neurodegener. 6, 81 (2011).
Yu, G., Zucchi, R., Ronca-Testoni, S. & Ronca, G. Protection of ischemic rat heart by dantrolene, an antagonist of the sarcoplasmic reticulum calcium release channel. Bas. Res. Cardiol 95, 137–143 (2000).
Javadov, S. A. et al. Protection of hearts from reperfusion injury by propofol is associated with inhibition of the mitochondrial permeability transition. Cardiovasc. Res. 45, 360–369 (2000).
Kourtis, N., Nikoletopoulou, V. & Tavernarakis, N. Small heat-shock proteins protect from heat-stroke-associated neurodegeneration. Nature 490, 213–218 (2012).
Artal-Sanz, M., Samara, C., Syntichaki, P. & Tavernarakis, N. Lysosomal biogenesis and function is critical for necrotic cell death in Caenorhabditis elegans. J. Cell Biol. 173, 231–239 (2006).
Aits, S. & Jäättelä, M. Lysosomal cell death at a glance. J. Cell Sci. 126, 1905–1912 (2013).
Kagedal, K., Zhao, M., Svensson, I. & Brunk, U. T. Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem. J. 359, 335–343 (2001).
Feofanov, A. et al. Cancer cell injury by cytotoxins from cobra venom is mediated through lysosomal damage. Biochem. J. 390, 11–18 (2005).
Malagoli, D., Marchesini, E. & Ottaviani, E. Lysosomes as the target of yessotoxin in invertebrate and vertebrate cell lines. Toxicol. Lett. 167, 75–83 (2006).
Kreuzaler, P. et al. Stat3 controls lysosomal-mediated cell death in vivo. Nature Cell Biol. 13, 303–309 (2011).
Vanlangenakker, N., Bertrand, M., Bogaert, P., Vandenabeele, P. & Vanden Berghe, T. TNF-induced necroptosis in L929 cells is tightly regulated by multiple TNFR1 complex I and II members. Cell Death Dis. 2, e230 (2011).
Upton, J., Kaiser, W. & Mocarski, E. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7, 302–313 (2010).
Vince, J. et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity 36, 215–227 (2012).
Jamison, J., Gilloteaux, J., Taper, H., Calderon, P. & Summers, J. Autoschizis: a novel cell death. Biochem. Pharmacol. 63, 1773–1783 (2002).
Gilloteaux, J. et al. Cell damage and death by autoschizis in human bladder (RT4) carcinoma cells resulting from treatment with ascorbate and menadione. Ultrastruct. Pathol. 34, 140–160 (2010).
Liu, Y. et al. Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc. Natl Acad. Sci. USA http://dx.doi.org/10.1073/pnas.1319661110 (2013).
Wilson, C. & Browning, J. Death of HT29 adenocarcinoma cells induced by TNF family receptor activation is caspase-independent and displays features of both apoptosis and necrosis. Cell Death Differ. 9, 1321–1333 (2002).
Tenev, T. et al. The ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell 43, 432–448 (2011).
Feoktistova, M. et al. cIAPs block ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell 43, 449–463 (2011).
Ch'en, I. L. et al. Antigen-mediated T cell expansion regulated by parallel pathways of death. Proc. Natl Acad. Sci. USA 105, 17463–17468 (2008).
Zou, J. et al. Poly IC triggers a cathepsin D− and IPS-1-dependent pathway to enhance cytokine production and mediate dendritic cell necroptosis. Immunity 38, 717–728 (2013).
Han, W. et al. Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol. Cancer Ther. 6, 1641–1649 (2007).
Huang, C. et al. Shikonin kills glioma cells through necroptosis mediated by RIP-1. PLoS ONE 8, e66326 (2013).
Basit, F., Cristofanon, S. & Fulda, S. Obatoclax (GX15-070) triggers necroptosis by promoting the assembly of the necrosome on autophagosomal membranes. Cell Death Differ. 20, 1161–1173 (2013).
Thapa, R. et al. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc. Natl Acad. Sci. USA 110, 18 (2013).
Williams, B. PKR; a sentinel kinase for cellular stress. Oncogene 18, 6112–6120 (1999).
Matsumura, H. et al. Necrotic death pathway in fas receptor signaling. J. Cell Biol. 151, 1247–1256 (2000).
Kaiser, W. J. et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368–372 (2011).
Oberst, A. et al. Catalytic activity of the caspase-8–FLIPL complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 (2011).
Welz, P. S. et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 477, 330–334 (2011).
Zhang, H. et al. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 471, 373–376 (2011).
Dillon, C. et al. Survival function of the FADD–CASPASE-8–cFLIPL complex. Cell Rep. 1, 401–407 (2012).
You, Z. et al. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 28, 1564–1573 (2008).
Lim, S. Y., Davidson, S. M., Mocanu, M. M., Yellon, D. M. & Smith, C. C. The cardioprotective effect of necrostatin requires the cyclophilin-D component of the mitochondrial permeability transition pore. Cardiovasc. Drugs Ther. 21, 467–469 (2007).
Rosenbaum, D. M. et al. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J. Neurosci. Res. 88, 1569–1576 (2010).
Trichonas, G. et al. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc. Natl Acad. Sci. USA 107, 21695–21700 (2010).
Murakami, Y. et al. Receptor interacting protein kinase mediates necrotic cone but not rod cell death in a mouse model of inherited degeneration. Proc. Natl Acad. Sci. USA 109, 14598–14603 (2012).
Linkermann, A. et al. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 81, 751–761 (2012).
Bonnet, M. C. et al. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity 35, 572–582 (2011).
Robinson, N. et al. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nature Immunol. 13, 954–962 (2012).
Duprez, L. et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity 35, 908–918 (2011).
Lin, J. et al. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep. 3, 200–210 (2013).
Colbert, L. et al. Pronecrotic mixed lineage kinase domain-like protein expression is a prognostic biomarker in patients with early-stage resected pancreatic adenocarcinoma. Cancer 119, 3148–3155 (2013).
Cai, Z. et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nature Cell Biol. 16, 55–65 (2014).
Chen, X. et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Research 24, 105–121 (2014).
Doitsh, G. et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature http://dx.doi.org/10.1038/nature12940 (2013).
Acknowledgements
The authors apologize to colleagues whose work was not discussed in this Opinion owing to space limitations. Research in the group of P.V. is supported by Belgian grants (Interuniversity Attraction Poles, IAP 7/32), Flemish grants (Research Foundation Flanders (FWO) G.0875.11, FWO G.0973.11, FWO G.0A45.12N, FWO G.0787.13N, Methusalem grant - BOF09/01M00709), Ghent University grants (MRP, GROUP-ID consortium, Belgium), a grant from the Foundation against Cancer, F94 and grants from the Flanders Institute for Biotechnology (VIB). H.W. is funded by a Cancer Research UK programme grant, an European Research Council (ERC) Advanced Grant and a Wellcome Trust Senior Investigator Award, UK. T.V.B. holds a postdoctoral fellowship from the FWO. A.L. is funded by the German Society for Nephrology, Novartis, Pfizer, Fresenius and the Hans-Werner Jackstädt Stiftung. S.J.L. is an omics@vib postdoctoral fellow at the VIB, co-funded by the Marie Curie COFUND initiative.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
H.W. is co-founder and shareholder of Apogenix GmbH (Heidelberg, Germany), a biotech company developing innovative drugs that target cell death and inflammatory pathways. T.V.B., A.L., S.J.L. and P.V. declare no competing interests.
Rights and permissions
About this article
Cite this article
Berghe, T., Linkermann, A., Jouan-Lanhouet, S. et al. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 15, 135–147 (2014). https://doi.org/10.1038/nrm3737
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3737
This article is cited by
-
Immunogenic cell death in cancer: targeting necroptosis to induce antitumour immunity
Nature Reviews Cancer (2024)
-
Regulated Necrosis in Glaucoma: Focus on Ferroptosis and Pyroptosis
Molecular Neurobiology (2024)
-
Global trends in PANoptosis research: bibliometrics and knowledge graph analysis
Apoptosis (2024)
-
Harnessing TRAIL-induced cell death for cancer therapy: a long walk with thrilling discoveries
Cell Death & Differentiation (2023)
-
Small extracellular vesicles delivering lncRNA WAC-AS1 aggravate renal allograft ischemia‒reperfusion injury by inducing ferroptosis propagation
Cell Death & Differentiation (2023)