Key Points
-
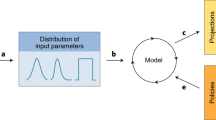
We show how mathematical models of infectious diseases can be used to understand the dynamics of outbreaks and epidemics, design effective interventions and make informed health policy decisions. To illustrate the usefulness of mathematical models to the microbiology and medical communities, the construction and application of a simple transmission model of an emerging pathogen — community-acquired meticillin-resistant Staphylococcus aureus (CA-MRSA) — is explained.
-
We show how to construct a model of a large (>8,000 reported cases) on-going outbreak of CA-MRSA in the Los Angeles County Jail (LACJ). We explain how to parameterize the model and reconstruct the outbreak. We then demonstrate how to use the model to assess the severity of the outbreak, predict the epidemiological consequences of a catastrophic outbreak and design effective interventions for outbreak control.
-
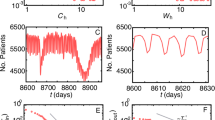
By using the model it was determined that the within-jail transmission was not high enough to sustain the outbreak, and that it was only sustained because of the continuous inflow of colonized and infected individuals from the community.
-
The modelling also revealed that the LACJ outbreak, although large, is not catastrophic, but would have become catastrophic if inmates had been incarcerated for more than a couple of months. In a worst-case scenario, the prevalence of infection within the jail would have increased to approximately 40%, and several thousand colonized and infected inmates would have been released into the community each month.
-
These analyses revealed that the outbreak could have been controlled if a large-scale highly effective screening programme of entering inmates had been set up to identify both colonized individuals and infected individuals.
-
The development of more complex transmission models, using the simple model as a platform, is discussed. This would allow investigators to gain additional quantitative insights into outbreak dynamics.
Abstract
To illustrate the usefulness of mathematical models to the microbiology and medical communities, we explain how to construct and apply a simple transmission model of an emerging pathogen. We chose to model, as a case study, a large (>8,000 reported cases) on-going outbreak of community-acquired meticillin-resistant Staphylococcus aureus (CA-MRSA) in the Los Angeles County Jail. A major risk factor for CA-MRSA infection is incarceration. Here, we show how to design a within-jail transmission model of CA-MRSA, parameterize the model and reconstruct the outbreak. The model is then used to assess the severity of the outbreak, predict the epidemiological consequences of a catastrophic outbreak and design effective interventions for outbreak control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Anderson, R. M. & May, R. M. Infectious Diseases of Humans: Dynamics and Control (Oxford University Press, Oxford, 1992).
Bernoulli, D. Essai d'une nouvelle analyse de la mortalite causee par la petite verole. Mem. Math. Phy. Acad. Roy. Sci. Paris, 1–45 (1766).
Blower, S. & Bernoulli, D. An attempt at a new analysis of the mortality caused by smallpox and of the advantages of inoculation to prevent it. Rev. Med. Virol. 14, 275–288 (2004).
Hota, B. et al. Community-associated meticillin-resistant Staphylococcus aureus skin and soft tissue infections at a public hospital: do public housing and incarceration amplify transmission? Arch. Intern. Med. 167, 1026–1033 (2007).
Special Studies Report 33–37 (Acute Communicable Disease Control, Los Angeles, 2005).
Grundmann, H., Aires- de-Sousa, M., Boyce, J. & Tiemersma, E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 368, 874–885 (2006).
Chambers, H. F. The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 7, 178–182 (2001).
Meticillin-resistant Staphylococcus aureus infections in correctional facilities — Georgia, California, and Texas, 2001–2003. Morb. Mortal. Wkly Rep. 52, 992–996 (2003).
Chambers, H. F. Community-associated MRSA — resistance and virulence converge. N. Engl. J. Med. 352, 1485–1487 (2005).
Charlebois, E. D. et al. Origins of community strains of meticillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 39, 47–54 (2004).
Naimi, T. S. et al. Epidemiology and clonality of community-acquired meticillin-resistant Staphylococcus aureus in Minnesota, 1996–1998. Clin. Infect. Dis. 33, 990–996 (2001).
Naimi, T. S. et al. Comparison of community- and health care-associated meticillin-resistant Staphylococcus aureus infection. JAMA 290, 2976–2984 (2003).
Salgado, C. D., Farr, B. M. & Calfee, D. P. Community-acquired meticillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin. Infect. Dis. 36, 131–139 (2003).
Baba, T. et al. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359, 1819–1827 (2002).
Diep, B. A., Sensabaugh, G. F., Somboona, N. S., Carleton, H. A. & Perdreau-Remington, F. Widespread skin and soft-tissue infections due to two meticillin-resistant Staphylococcus aureus strains harboring the genes for Panton-Valentine leucocidin. J. Clin. Microbiol. 42, 2080–2084 (2004).
Fridkin, S. K. et al. Meticillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352, 1436–1444 (2005).
McDougal, L. K. et al. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41, 5113–5120 (2003).
Vandenesch, F. et al. Community-acquired meticillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9, 978–984 (2003).
Four pediatric deaths from community-acquired meticillin-resistant Staphylococcus aureus — Minnesota and North Dakota, 1997–1999. JAMA 282, 1123–1125 (1999).
Miller, L. G. et al. Necrotizing fasciitis caused by community-associated meticillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 352, 1445–1453 (2005).
Austin, D. J. & Anderson, R. M. Studies of antibiotic resistance within the patient, hospitals and the community using simple mathematical models. Philos. Trans R. Soc. Lond. B Biol. Sci. 354, 721–738 (1999).
Bootsma, M. C., Diekmann, O. & Bonten, M. J. Controlling meticillin-resistant Staphylococcus aureus: quantifying the effects of interventions and rapid diagnostic testing. Proc. Natl Acad. Sci. USA 103, 5620–5625 (2006).
Cooper, B. S., Medley, G. F. & Scott, G. M. Preliminary analysis of the transmission dynamics of nosocomial infections: stochastic and management effects. J. Hosp. Infect. 43, 131–147 (1999).
D'Agata, E. M., Webb, G. & Horn, M. A mathematical model quantifying the impact of antibiotic exposure and other interventions on the endemic prevalence of vancomycin-resistant enterococci. J. Infect. Dis. 192, 2004–2011 (2005).
Forrester, M. & Pettitt, A. N. Use of stochastic epidemic modeling to quantify transmission rates of colonization with meticillin-resistant Staphylococcus aureus in an intensive care unit. Infect. Control Hosp. Epidemiol. 26, 598–606 (2005).
Forrester, M. L., Pettitt, A. N. & Gibson, G. J. Bayesian inference of hospital-acquired infectious diseases and control measures given imperfect surveillance data. Biostatistics 8, 383–401 (2007).
Hotchkiss, J. R., Strike, D. G., Simonson, D. A., Broccard, A. F. & Crooke, P. S. An agent-based and spatially explicit model of pathogen dissemination in the intensive care unit. Crit. Care Med. 33, 168–176 (2005).
McBryde, E. S., Pettitt, A. N. & McElwain, D. L. A stochastic mathematical model of meticillin resistant Staphylococcus aureus transmission in an intensive care unit: predicting the impact of interventions. J. Theor. Biol. 245, 470–481 (2007).
Raboud, J. et al. Modeling transmission of meticillin-resistant Staphylococcus aureus among patients admitted to a hospital. Infect. Control Hosp. Epidemiol. 26, 607–615 (2005).
Robotham, J. V., Jenkins, D. R. & Medley, G. F. Screening strategies in surveillance and control of meticillin-resistant Staphylococcus aureus (MRSA). Epidemiol. Infect. 135, 328–342 (2007).
Sebille, V., Chevret, S. & Valleron, A. J. Modeling the spread of resistant nosocomial pathogens in an intensive-care unit. Infect. Control Hosp. Epidemiol. 18, 84–92 (1997).
Sebille, V. & Valleron, A. J. A computer simulation model for the spread of nosocomial infections caused by multidrug-resistant pathogens. Comput. Biomed. Res. 30, 307–322 (1997).
Smith, D. L., Levin, S. A. & Laxminarayan, R. Strategic interactions in multi-institutional epidemics of antibiotic resistance. Proc. Natl Acad. Sci. USA 102, 3153–3158 (2005).
Wang, Y. C. & Lipsitch, M. Upgrading antibiotic use within a class: tradeoff between resistance and treatment success. Proc. Natl Acad. Sci. USA 103, 9655–9660 (2006).
Outbreaks of community-associated meticillin-resistant Staphylococcus aureus skin infections — Los Angeles County, California, 2002–2003. Morb. Mortal. Wkly Rep. 52, 88 (2003).
Kajita, E. MS in Biomathematics (University of California, Los Angeles, 2004).
Ngo, V., Tadesse, M. & Bancroft, E. in Proceedings of the 15th Annual Meeting of the Society for Healthcare Epidemiology of America (Los Angeles, Calfornia, 2005).
Pan, E. S. et al. Increasing prevalence of meticillin-resistant Staphylococcus aureus infection in California jails. Clin. Infect. Dis. 37, 1384–1388 (2003).
Tenover, F. C. et al. Characterization of a strain of community-associated meticillin-resistant Staphylococcus aureus widely disseminated in the United States. J. Clin. Microbiol. 44, 108–118 (2006).
Meticillin-resistant Staphylococcus aureus skin or soft tissue infections in a state prison — Mississippi, 2000. Morb. Mortal. Wkly Rep. 50, 919–922 (2001).
Turabelidze, G. et al. Personal hygiene and meticillin-resistant Staphylococcus aureus infection. Emerg. Infect. Dis. 12, 422–427 (2006).
Wootton, S. H. et al. Intervention to reduce the incidence of meticillin-resistant Staphylococcus aureus skin infections in a correctional facility in Georgia. Infect. Control Hosp. Epidemiol. 25, 402–407 (2004).
Baillargeon, J., Kelley, M. F., Leach, C. T., Baillargeon, G. & Pollock, B. H. Meticillin-resistant Staphylococcus aureus infection in the Texas prison system. Clin. Infect. Dis. 38, e92–e95 (2004).
Blower, S. & Dowlatabadi, H. Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. Int. Stat. Rev. 62, 229–243 (1994).
Blower, S. M., Aschenbach, A. N., Gershengorn, H. B. & Kahn, J. O. Predicting the unpredictable: transmission of drug-resistant HIV. Nature Med. 7, 1016–1020 (2001).
Blower, S. M. & Chou, T. Modeling the emergence of the 'hot zones': tuberculosis and the amplification dynamics of drug resistance. Nature Med. 10, 1111–1116 (2004).
Blower, S. M., Gershengorn, H. B. & Grant, R. M. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science 287, 650–654 (2000).
Blower, S. M., Koelle, K., Kirschner, D. E. & Mills, J. Live attenuated HIV vaccines: predicting the tradeoff between efficacy and safety. Proc. Natl Acad. Sci. USA 98, 3618–3623 (2001).
Sanchez, M. A. & Blower, S. M. Uncertainty and sensitivity analysis of the basic reproductive rate. Tuberculosis as an example. Am. J. Epidemiol. 145, 1127–1137 (1997).
van den Driessche, P. & Watmough, J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 180, 29–48 (2002).
Acknowledgements
The authors acknowledge J. Boscardin, R. Breban, N. Freimer, V. Supervie, R. Vardavas, D. Wilson and all the staff at the Los Angeles County Jail and the Los Angeles County Department of Public Health. T. Pylko is also acknowledged for helpful clinical discussions during the course of this research. Financial support was received from the National Institute of Allergy and Infectious Diseases in the National Institutes of Health (R01 AI04 935).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Genome Project
FURTHER INFORMATION
Glossary
- Catastrophic outbreak
-
An extremely large outbreak in a confined population that may be caused by the synergistic interaction of two processes: a high level of transmission and a large inflow of infectious individuals into the transmission site.
- Prevalence
-
The number of infected individuals at a specific time.
- Nurse cohorting
-
Reducing the contact of nurses with a large group of patients by assigning specific groups of nurses to the care of only a subset of patients. This intervention reduces the interaction of nurses with patients and is therefore expected to reduce nurse–patient transmission.
- Title 15
-
Title 15 deals with “Miscellaneous Crimes” and is part of the California Penal Code.
- Incidence
-
The number of newly infected individuals per unit of time.
- Uncertainty analysis
-
An analysis in which the variation in the value of an outcome variable is determined. The variability that is observed in the outcome variable is due to the uncertainty in estimating the exact value of each parameter in the model.
- Simulation
-
An analysis of a model that is carried out with a computer. The model is programmed using a computer language and then run using specific parameter values. Each run of the model is called a simulation, or a scenario.
Rights and permissions
About this article
Cite this article
Kajita, E., Okano, J., Bodine, E. et al. Modelling an outbreak of an emerging pathogen. Nat Rev Microbiol 5, 700–709 (2007). https://doi.org/10.1038/nrmicro1660
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1660
This article is cited by
-
Hand hygiene improvement or antibiotic restriction to control the household transmission of extended-spectrum β-lactamase-producing Escherichia coli: a mathematical modelling study
Antimicrobial Resistance & Infection Control (2020)
-
Dynamic contact networks of patients and MRSA spread in hospitals
Scientific Reports (2020)
-
A systematic review of transmission dynamic studies of methicillin-resistant Staphylococcus aureus in non-hospital residential facilities
BMC Infectious Diseases (2018)
-
Empfehlungen zur Prävention und Kontrolle von Methicillin-resistenten Staphylococcus aureus-Stämmen (MRSA) in medizinischen und pflegerischen Einrichtungen
Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (2014)
-
Molecular Epidemiology of Community-Associated Methicillin-resistant Staphylococcus aureus in the genomic era: a Cross-Sectional Study
Scientific Reports (2013)