Key Points
-
Malaria has devastating consequences: it strikes over 250 million people worldwide and kills approximately 1 million people each year, many of whom are children under 5 years of age.
-
Malaria can be prevented by interventions focused on breaking the cycle of transmission, either by eliminating the mosquito (through the use of insecticides) or preventing bites (through the use of insecticide-treated bed nets).
-
It can also be treated through the use of antimalarial drugs. Drug resistance, however, remains the biggest threat to current drug efficacy. The former mainstays of antimalarial chemotherapy, chloroquine and sulfadoxine–pyrimethamine, have been rendered ineffective for the treatment of Plasmodium falciparum malaria by the emergence and spread of drug-resistant parasites.
-
Almost all malaria-endemic regions have switched to artemisinin (ART)-based combination therapies (ACTs) for the first-line treatment of P. falciparum malaria.
-
ACTs combine an ART semisynthetic derivative, which has a short half-life, with a longer-lasting partner drug. This results in sustained antimalarial pressure after the plasma concentrations of the ART derivatives have fallen below therapeutic levels
-
ACTs are discussed in terms of their modes of action and pharmacokinetic properties and the proposed mechanisms of resistance to them.
-
We summarize several therapeutic strategies that might decrease the emergence of drug resistance and present a perspective on the current ACT-based efforts to reduce the burden of malaria.
Abstract
Plasmodium falciparum resistance to chloroquine and sulphadoxine–pyrimethamine has led to the recent adoption of artemisinin-based combination therapies (ACTs) as the first line of treatment against malaria. ACTs comprise semisynthetic artemisinin derivatives paired with distinct chemical classes of longer acting drugs. These artemisinins are exceptionally potent against the pathogenic asexual blood stages of Plasmodium parasites and also act on the transmissible sexual stages. These combinations increase the rates of clinical and parasitological cures and decrease the selection pressure for the emergence of antimalarial resistance. This Review article discusses our current knowledge about the mode of action of ACTs, their pharmacological properties and the proposed mechanisms of drug resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wellems, T. E. & Plowe, C. V. Chloroquine-resistant malaria. J. Infect. Dis. 184, 770–776 (2001).
Gregson, A. & Plowe, C. V. Mechanisms of resistance of malaria parasites to antifolates. Pharmacol. Rev. 57, 117–145 (2005).
Wongsrichanalai, C., Pickard, A. L., Wernsdorfer, W. H. & Meshnick, S. R. Epidemiology of drug-resistant malaria. Lancet Infect. Dis. 2, 209–218 (2002).
Snow, R. W., Guerra, C. A., Noor, A. M., Myint, H. Y. & Hay, S. I. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434, 214–217 (2005).
Talisuna, A. O., Okello, P. E., Erhart, A., Coosemans, M. & D'Alessandro, U. Intensity of malaria transmission and the spread of Plasmodium falciparum resistant malaria: a review of epidemiologic field evidence. Am. J. Trop. Med. Hyg. 77, 170–180 (2007).
Feachem, R. & Sabot, O. A new global malaria eradication strategy. Lancet 371, 1633–1635 (2008). Discusses the need to coordinate a global strategy to progressively eliminate malaria.
Jiang, J. B., Li, G. Q., Guo, X. B., Kong, Y. C. & Arnold, K. Antimalarial activity of mefloquine and qinghaosu. Lancet 2, 285–288 (1982).
White, N. J. Qinghaosu (artemisinin): the price of success. Science 320, 330–334 (2008). Reviews artemisinins, including a historical perspective, their pharmacological properties, their clinical efficacy and initial evidence of emerging resistance.
Ro, D. K. et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440, 940–943 (2006).
German, P. I. & Aweeka, F. T. Clinical pharmacology of artemisinin-based combination therapies. Clin. Pharmacokinet. 47, 91–102 (2008).
Golenser, J., Waknine, J. H., Krugliak, M., Hunt, N. H. & Grau, G. E. Current perspectives on the mechanism of action of artemisinins. Int. J. Parasitol. 36, 1427–1441 (2006). Summarizes a large and sometimes conflicting body of investigations into the mode of action of artemisinins, their metabolism, suggested mechanisms of resistance and adverse events.
Hartwig, C. L. et al. Accumulation of artemisinin trioxane derivatives within neutral lipids of Plasmodium falciparum malaria parasites is endoperoxide-dependent. Biochem. Pharmacol. 77, 322–336 (2009).
Robert, A., Benoit-Vical, F., Claparols, C. & Meunier, B. The antimalarial drug artemisinin alkylates heme in infected mice. Proc. Natl Acad. Sci. USA 102, 13676–13680 (2005).
Bousejra- El Garah, F., Claparols, C., Benoit-Vical, F., Meunier, B. & Robert, A. The antimalarial trioxaquine DU1301 alkylates heme in malaria-infected mice. Antimicrob. Agents Chemother. 52, 2966–2969 (2008).
Creek, D. J. et al. Relationship between antimalarial activity and heme alkylation for spiro- and dispiro-1,2,4-trioxolane antimalarials. Antimicrob. Agents Chemother. 52, 1291–1296 (2008).
Creek, D. J. et al. Stability of peroxide antimalarials in the presence of human hemoglobin. Antimicrob. Agents Chemother. 53, 3496–3500 (2009).
Krungkrai, S. R. & Yuthavong, Y. The antimalarial action on Plasmodium falciparum of qinghaosu and artesunate in combination with agents which modulate oxidant stress. Trans. R. Soc. Trop. Med. Hyg. 81, 710–714 (1987).
Asawamahasakda, W., Ittarat, I., Pu, Y. M., Ziffer, H. & Meshnick, S. R. Reaction of antimalarial endoperoxides with specific parasite proteins. Antimicrob. Agents Chemother. 38, 1854–1858 (1994).
Kannan, R., Kumar, K., Sahal, D., Kukreti, S. & Chauhan, V. S. Reaction of artemisinin with haemoglobin: implications for antimalarial activity. Biochem. J. 385, 409–418 (2005).
Wu, Y. How might qinghaosu (artemisinin) and related compounds kill the intraerythrocytic malaria parasite? A chemist's view. Acc. Chem. Res. 35, 255–259 (2002).
Stocks, P. A. et al. Evidence for a common non-heme chelatable-iron-dependent activation mechanism for semisynthetic and synthetic endoperoxide antimalarial drugs. Angew. Chem. Int. Edn Engl. 46, 6278–6283 (2007).
del Pilar Crespo, M. et al. Artemisinin and a series of novel endoperoxide antimalarials exert early effects on digestive vacuole morphology. Antimicrob. Agents Chemother. 52, 98–109 (2008).
Bhisutthibhan, J. & Meshnick, S. R. Immunoprecipitation of [3H]-dihydroartemisinin translationally controlled tumor protein (TCTP) adducts from Plasmodium falciparum-infected erythrocytes by using anti-TCTP antibodies. Antimicrob. Agents Chemother. 45, 2397–2399 (2001).
Olliaro, P. L., Haynes, R. K., Meunier, B. & Yuthavong, Y. Possible modes of action of the artemisinin-type compounds. Trends Parasitol. 17, 122–126 (2001).
Eckstein-Ludwig, U. et al. Artemisinins target the SERCA of Plasmodium falciparum. Nature 424, 957–961 (2003).
Jambou, R. et al. Resistance of Plasmodium falciparum field isolates to in vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet 366, 1960–1963 (2005).
Dahlstrom, S. et al. Diversity of the sarco/endoplasmic reticulum Ca2+-ATPase orthologue of Plasmodium falciparum (PfATP6). Infect. Genet. Evol. 8, 340–345 (2008).
Valderramos, S. G. & Fidock, D. A. Transporters involved in resistance to antimalarial drugs. Trends Pharmacol. Sci. 27, 594–601 (2006).
Price, R. N. et al. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet 364, 438–447 (2004). Demonstrates that amplification of pfmdr1 is a major mediator of resistance to mefloquine in P. falciparum malaria.
Sidhu, A. B. et al. Decreasing pfmdr1 copy number in Plasmodium falciparum malaria heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine, and artemisinin. J. Infect. Dis. 194, 528–535 (2006).
Raj, D. K. et al. Disruption of a Plasmodium falciparum multidrug resistance-associated protein (PfMRP) alters its fitness and transport of antimalarial drugs and glutathione. J. Biol. Chem. 284, 7687–7696 (2009).
Noedl, H., Socheat, D. & Satimai, W. Artemisinin-resistant malaria in Asia. N. Engl. J. Med. 361, 540–541 (2009).
Dondorp, A. M. et al. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361, 455–467 (2009). Provides clinical evidence for delayed parasite clearance times in patients with a P. falciparum infection who are treated with AS in western Cambodia, and calls for urgent containment methods to halt the spread of resistance.
Wootton, J. C. et al. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature 418, 320–323 (2002).
Roper, C. et al. Intercontinental spread of pyrimethamine-resistant malaria. Science 305, 1124 (2004).
Klein, E. Y., Smith, D. L., Boni, M. F. & Laxminarayan, R. Clinically immune hosts as a refuge for drug-sensitive malaria parasites. Malar. J. 7, 67 (2008).
Rathod, P. K., McErlean, T. & Lee, P.-C. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc. Natl Acad. Sci. USA 94, 9389–9393 (1997). Provides compelling evidence that some P. falciparum strains harbour the ability to rapidly acquire antimalarial drug resistance, termed the 'accelerated resistance to multiple drugs' phenotype.
Ekland, E. H. & Fidock, D. A. Advances in understanding the genetic basis of antimalarial drug resistance. Curr. Opin. Microbiol. 10, 363–370 (2007). Summarizes recent developments in genetic and genomic tools to explore theresistance of Plasmodium spp. to antimalarial drugs.
Dharia, N. V. et al. Use of high-density tiling microarrays to identify mutations globally and elucidate mechanisms of drug resistance in Plasmodium falciparum. Genome Biol. 10, R21 (2009). Describes a rapid method, based on hybridizations of a P. falciparum 4.8-million-feature tiled array that covers 90% of coding regions, to identify SNPs and copy number variations in drug-pressured mutant parasites.
Kozarewa, I. et al. Amplification-free Illumina sequencing-library preparation facilitates improved mapping and assembly of (G+C)-biased genomes. Nature Methods 6, 291–295 (2009).
Afonso, A. et al. Malaria parasites can develop stable resistance to artemisinin but lack mutations in candidate genes atp6 (encoding the sarcoplasmic and endoplasmic reticulum Ca2+ ATPase), tctp, mdr1, and cg10. Antimicrob. Agents Chemother. 50, 480–489 (2006).
Hunt, P. et al. Gene encoding a deubiquitinating enzyme is mutated in artesunate- and chloroquine-resistant rodent malaria parasites. Mol. Microbiol. 65, 27–40 (2007).
Woodrow, C. J. & Krishna, S. Antimalarial drugs: recent advances in molecular determinants of resistance and their clinical significance. Cell Mol. Life Sci. 63, 1586–1596 (2006).
Bukirwa, H. & Critchley, J. Sulfadoxine-pyrimethamine plus artesunate versus sulfadoxine-pyrimethamine plus amodiaquine for treating uncomplicated malaria. Cochrane Database Syst. Rev. 2006, CD004966 (2006).
Sullivan, D. J., Jr, Matile, H., Ridley, R. G. & Goldberg, D. E. A common mechanism for blockade of heme polymerization by antimalarial quinolines. J. Biol. Chem. 273, 31103–31107 (1998). Demonstrates that antimalarial–haem complex formation is potentially a common mechanism of drug action.
Rohrbach, P. et al. Genetic linkage of pfmdr1 with food vacuolar solute import in Plasmodium falciparum. EMBO J. 25, 3000–3011 (2006).
Alker, A. P. et al. Pfmdr1 and in vivo resistance to artesunate-mefloquine in falciparum malaria on the Cambodian-Thai border. Am. J. Trop. Med. Hyg. 76, 641–647 (2007).
Nosten, F. et al. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet 356, 297–302 (2000).
Nosten, F. et al. Treatment of multidrug-resistant Plasmodium falciparum malaria with 3-day artesunate-mefloquine combination. J. Infect. Dis. 170, 971–977 (1994).
Jansen, F. H. et al. Assessment of the relative advantage of various artesunate-based combination therapies by a multi-treatment Bayesian random-effects meta-analysis. Am. J. Trop. Med. Hyg. 77, 1005–1009 (2007).
Fitch, C. D. Ferriprotoporphyrin IX, phospholipids, and the antimalarial actions of quinoline drugs. Life Sci. 74, 1957–1972 (2004).
Hassan Alin, M., Bjorkman, A. & Wernsdorfer, W. H. Synergism of benflumetol and artemether in Plasmodium falciparum. Am. J. Trop. Med. Hyg. 61, 439–445 (1999).
Ezzet, F., Mull, R. & Karbwang, J. Population pharmacokinetics and therapeutic response of CGP 56697 (artemether + benflumetol) in malaria patients. Br. J. Clin. Pharmacol. 46, 553–561 (1998).
Ezzet, F., van Vugt, M., Nosten, F., Looareesuwan, S. & White, N. J. Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrob. Agents Chemother. 44, 697–704 (2000).
Checchi, F. et al. Supervised versus unsupervised antimalarial treatment with six-dose artemether-lumefantrine: pharmacokinetic and dosage-related findings from a clinical trial in Uganda. Malar. J. 5, 59 (2006).
Bloland, P. B., Ettling, M. & Meek, S. Combination therapy for malaria in Africa: hype or hope? Bull. World Health Organ. 78, 1378–1388 (2000).
Price, R. N. et al. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin. Infect. Dis. 42, 1570–1577 (2006).
Sisowath, C. et al. In vivo selection of Plasmodium falciparum pfmdr1 86N coding alleles by artemether-lumefantrine (Coartem). J. Infect. Dis. 191, 1014–1017 (2005).
Dokomajilar, C., Nsobya, S. L., Greenhouse, B., Rosenthal, P. J. & Dorsey, G. Selection of Plasmodium falciparum pfmdr1 alleles following therapy with artemether-lumefantrine in an area of Uganda where malaria is highly endemic. Antimicrob. Agents Chemother. 50, 1893–1895 (2006).
Sisowath, C. et al. The role of pfmdr1 in Plasmodium falciparum tolerance to artemether-lumefantrine in Africa. Trop. Med. Int. Health 12, 736–742 (2007).
Sisowath, C. et al. In vivo selection of Plasmodium falciparum parasites carrying the chloroquine-susceptible pfcrt K76 allele after treatment with artemether-lumefantrine in Africa. J. Infect. Dis. 199, 750–757 (2009).
Abdulla, S. et al. Efficacy and safety of artemether-lumefantrine dispersible tablets compared with crushed commercial tablets in African infants and children with uncomplicated malaria: a randomised, single-blind, multicentre trial. Lancet 372, 1819–1827 (2008). First clinical report of the dispersible formulation of ATM–LMF, showing excellent clinical efficacy in young African children with uncomplicated malaria. This paediatric formulation should substantially improve administration and dosing of this widely used ACT.
Naisbitt, D. J. et al. Metabolism-dependent neutrophil cytotoxicity of amodiaquine: a comparison with pyronaridine and related antimalarial drugs. Chem. Res. Toxicol. 11, 1586–1595 (1998).
Olliaro, P. & Mussano, P. Amodiaquine for treating malaria. Cochrane Database Syst. Rev. 2003, CD000016 (2003).
Gasasira, A. F. et al. High risk of neutropenia in HIV-infected children following treatment with artesunate plus amodiaquine for uncomplicated malaria in Uganda. Clin. Infect. Dis. 46, 985–991 (2008).
Pussard, E. et al. Disposition of monodesethylamodiaquine after a single oral dose of amodiaquine and three regimens for prophylaxis against Plasmodium falciparum malaria. Eur. J. Clin. Pharmacol. 33, 409–414 (1987).
White, N. J. et al. Pharmacokinetics of intravenous amodiaquine. Br. J. Clin. Pharmacol. 23, 127–135 (1987).
Legrand, E., Volney, B., Meynard, J. B., Mercereau-Puijalon, O. & Esterre, P. In vitro monitoring of Plasmodium falciparum drug resistance in French Guiana: a synopsis of continuous assessment from 1994 to 2005. Antimicrob. Agents Chemother. 52, 288–298 (2008).
de Dios, A. C., Casabianca, L. B., Kosar, A. & Roepe, P. D. Structure of the amodiaquine-FPIX mu oxo dimer solution complex at atomic resolution. Inorg. Chem. 43, 8078–8084 (2004).
Bray, P. G., Hawley, S. R., Mungthin, M. & Ward, S. A. Physicochemical properties correlated with drug resistance and the reversal of drug resistance in Plasmodium falciparum. Mol. Pharmacol. 50, 1559–1566 (1996).
Ochong, E. O., van den Broek, I. V., Keus, K. & Nzila, A. Short report: association between chloroquine and amodiaquine resistance and allelic variation in the Plasmodium falciparum multiple drug resistance 1 gene and the chloroquine resistance transporter gene in isolates from the upper Nile in southern Sudan. Am. J. Trop. Med. Hyg. 69, 184–187 (2003).
Holmgren, G. et al. Amodiaquine resistant Plasmodium falciparum malaria in vivo is associated with selection of pfcrt 76T and pfmdr1 86Y. Infect. Genet. Evol. 6, 309–314 (2006).
Pradines, B. et al. Prevalence of in vitro resistance to eleven standard or new antimalarial drugs among Plasmodium falciparum isolates from Pointe-Noire, Republic of the Congo. J. Clin. Microbiol. 44, 2404–2408 (2006).
Picot, S. et al. A systematic review and meta-analysis of evidence for correlation between molecular markers of parasite resistance and treatment outcome in falciparum malaria. Malar. J. 8, 89 (2009). Reviews many studies assessing the impact of known P. falciparum drug resistance determinants on the risk of treatment failure.
Faye, B. et al. Efficacy and tolerability of four antimalarial combinations in the treatment of uncomplicated Plasmodium falciparum malaria in Senegal. Malar. J. 6, 80 (2007).
Guthmann, J.-P. et al. High efficacy of two artemisinin-based combinations (artesunate + amodiaquine and artemether + lumefantrine) in Caala, Central Angola. Am. J. Trop. Med. Hyg. 75, 143–145 (2006).
Hasugian, A. R. et al. Dihydroartemisinin-piperaquine versus artesunate-amodiaquine: superior efficacy and posttreatment prophylaxis against multidrug-resistant Plasmodium falciparum and Plasmodium vivax malaria. Clin. Infect. Dis. 44, 1067–1074 (2007).
Hien, T. T. et al. Dihydroartemisinin-piperaquine against multidrug-resistant Plasmodium falciparum malaria in Vietnam: randomised clinical trial. Lancet 363, 18–22 (2004).
Vennerstrom, J. L. et al. Bisquinolines. 1. N,N-bis(7-chloroquinolin-4-yl)alkanediamines with potential against chloroquine-resistant malaria. J. Med. Chem. 35, 2129–2134 (1992).
Davis, T. M., Hung, T. Y., Sim, I. K., Karunajeewa, H. A. & Ilett, K. F. Piperaquine: a resurgent antimalarial drug. Drugs 65, 75–87 (2005).
Liu, C. et al. Pharmacokinetics of piperaquine after single and multiple oral administrations in healthy volunteers. Yakugaku Zasshi 127, 1709–1714 (2007).
Ashley, E. A. et al. A randomized, controlled study of a simple, once-daily regimen of dihydroartemisinin-piperaquine for the treatment of uncomplicated, multidrug-resistant falciparum malaria. Clin. Infect. Dis. 41, 425–432 (2005).
Smithuis, F. et al. Efficacy and effectiveness of dihydroartemisinin-piperaquine versus artesunate-mefloquine in falciparum malaria: an open-label randomised comparison. Lancet 367, 2075–2085 (2006).
Karunajeewa, H. et al. Safety evaluation of fixed combination piperaquine plus dihydroartemisinin (Artekin) in Cambodian children and adults with malaria. Br. J. Clin. Pharmacol. 57, 93–99 (2004).
Warhurst, D. C. & Duraisingh, M. T. Rational use of drugs against Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 95, 345–346 (2001).
Basco, L. K. & Ringwald, P. In vitro activities of piperaquine and other 4-aminoquinolines against clinical isolates of Plasmodium falciparum in Cameroon. Antimicrob. Agents Chemother. 47, 1391–1394 (2003).
Muangnoicharoen, S., Johnson, D. J., Looareesuwan, S., Krudsood, S. & Ward, S. A. Role of known molecular markers of resistance in the antimalarial potency of piperaquine and dihydroartemisinin in vitro. Antimicrob. Agents Chemother. 53, 1362–1366 (2009).
Vivas, L. et al. Anti-malarial efficacy of pyronaridine and artesunate in combination in vitro and in vivo. Acta Trop. 105, 222–228 (2008).
Basco, L. K., Ringwald, P., Franetich, J. F. & Mazier, D. Assessment of pyronaridine activity in vivo and in vitro against the hepatic stages of malaria in laboratory mice. Trans. R. Soc. Trop. Med. Hyg. 93, 651–652 (1999).
Ringwald, P., Meche, F. S. & Basco, L. K. Short report: effects of pyronaridine on gametocytes in patients with acute uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 61, 446–448 (1999).
Pagola, S., Stephens, P. W., Bohle, D. S., Kosar, A. D. & Madsen, S. K. The structure of malaria pigment β-haematin. Nature 404, 307–310 (2000). A landmark study that elucidated the structure of β-haematin, the proposed target of several antimalarials, including chloroquine. This structure revealed dimer linkages that are formed through reciprocal iron-carboxylate bonds, which are in turn linked into chains via hydrogen bonds in the haematin crystal.
Auparakkitanon, S., Chapoomram, S., Kuaha, K., Chirachariyavej, T. & Wilairat, P. Targeting of hematin by the antimalarial pyronaridine. Antimicrob. Agents Chemother. 50, 2197–2200 (2006).
Wu, L. J., Rabbege, J. R., Nagasawa, H., Jacobs, G. & Aikawa, M. Morphological effects of pyronaridine on malarial parasites. Am. J. Trop. Med. Hyg. 38, 30–36 (1988).
Chang, C., Lin-Hua, T. & Jantanavivat, C. Studies on a new antimalarial compound: pyronaridine. Trans. R. Soc. Trop. Med. Hyg. 86, 7–10 (1992).
Ringwald, P., Bickii, J. & Basco, L. Randomised trial of pyronaridine versus chloroquine for acute uncomplicated falciparum malaria in Africa. Lancet 347, 24–28 (1996).
Basco, L. K. & Ringwald, P. Molecular epidemiology of malaria in Yaounde, Cameroon. VII. Analysis of recrudescence and reinfection in patients with uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 63, 215–221 (2000).
Ramharter, M. et al. Fixed-dose pyronaridine-artesunate combination for treatment of uncomplicated falciparum malaria in pediatric patients in Gabon. J. Infect. Dis. 198, 911–919 (2008).
Price, R. N. et al. Vivax malaria: neglected and not benign. Am. J. Trop. Med. Hyg. 77, 79–87 (2007). Highlights the burden and impact of the often-overlooked disease, P. vivax malaria.
Ratcliff, A. et al. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet 369, 757–765 (2007).
Tjitra, E. et al. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 5, e128 (2008). Describes severe and fatal malaria resulting from P. vivax infections in an area with high-grade chloroquine resistance in both P. falciparum and P. vivax . Dispels earlier assumptions that P. vivax infection was rarely lethal.
Clark, R. L. et al. Developmental toxicity of artesunate and an artesunate combination in the rat and rabbit. Birth Defects Res. B. Dev. Reprod. Toxicol. 71, 380–394 (2004).
Brewer, T. G., Genovese, R. F., Newman, D. B. & Li, Q. Factors relating to neurotoxicity of artemisinin antimalarial drugs “listening to arteether”. Med. Trop. (Mars) 58, 22–27 (1998).
McGready, R. et al. Artemisinin antimalarials in pregnancy: a prospective treatment study of 539 episodes of multidrug-resistant Plasmodium falciparum. Clin. Infect. Dis. 33, 2009–2016 (2001).
Rijken, M. J. et al. Dihydroartemisinin–piperaquine rescue treatment of multidrug-resistant Plasmodium falciparum malaria in pregnancy: a preliminary report. Am. J. Trop. Med. Hyg. 78, 543–545 (2008).
White, N. J., McGready, R. M. & Nosten, F. H. New medicines for tropical diseases in pregnancy: catch-22. PLoS Med. 5, e133 (2008). Discusses the need for further studies on the use and evaluation of medicines for treatment of diseases during pregnancy.
WHO. World malaria report 2008 (WHO, Geneva, 2008).
Laufer, M. K., Djimde, A. A. & Plowe, C. V. Monitoring and deterring drug-resistant malaria in the era of combination therapy. Am. J. Trop. Med. Hyg. 77, 160–169 (2007).
Vestergaard, L. S. & Ringwald, P. Responding to the challenge of antimalarial drug resistance by routine monitoring to update national malaria treatment policies. Am. J. Trop. Med. Hyg. 77, 153–159 (2007).
Sibley, C. H., Barnes, K. I., Watkins, W. M. & Plowe, C. V. A network to monitor antimalarial drug resistance: a plan for moving forward. Trends Parasitol. 24, 43–48 (2008). Provides the rationale for the creation of WWARN, which is developing a group of open-access databases to aid antimalarial drug treatment and prevention decisions.
Newton, P. N. et al. A collaborative epidemiological investigation into the criminal fake artesunate trade in South East Asia. PLoS Med. 5, e32 (2008).
Bate, R., Coticelli, P., Tren, R. & Attaran, A. Antimalarial drug quality in the most severely malarious parts of Africa — a six country study. PLoS ONE 3, e2132 (2008).
Fidock, D. A., Eastman, R. T., Ward, S. A. & Meshnick, S. R. Recent highlights in antimalarial drug resistance and chemotherapy research. Trends Parasitol. 24, 537–544 (2008).
Hay, S. I., Smith, D. L. & Snow, R. W. Measuring malaria endemicity from intense to interrupted transmission. Lancet Infect. Dis. 8, 369–378 (2008).
Moon, S., Perez Casas, C., Kindermans, J. M., de Smet, M. & von Schoen-Angerer, T. Focusing on quality patient care in the new global subsidy for malaria medicines. PLoS Med. 6, e1000106 (2009).
Hay, S. I., Rogers, D. J., Toomer, J. F. & Snow, R. W. Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: literature survey, internet access and review. Trans. R. Soc. Trop. Med. Hyg. 94, 113–127 (2000).
Smith, D. L., Dushoff, J. & McKenzie, F. E. The risk of a mosquito-borne infection in a heterogeneous environment. PLoS Biol. 2, e368 (2004).
Coluzzi, M. The clay feet of the malaria giant and its African roots: hypotheses and inferences about origin, spread and control of Plasmodium falciparum. Parassitologia 41, 277–283 (1999).
Obsomer, V., Defourny, P. & Coosemans, M. The Anopheles dirus complex: spatial distribution and environmental drivers. Malar. J. 6, 26 (2007).
Struik, S. S. & Riley, E. M. Does malaria suffer from lack of memory? Immunol. Rev. 201, 268–290 (2004).
Breman, J. G., Alilio, M. S. & Mills, A. Conquering the intolerable burden of malaria: what's new, what's needed: a summary. Am. J. Trop. Med. Hyg. 71, 1–15 (2004).
Hastings, I. M. & Watkins, W. M. Intensity of malaria transmission and the evolution of drug resistance. Acta Trop. 94, 218–229 (2005).
Williams, T. N. Human red blood cell polymorphisms and malaria. Curr. Opin. Microbiol. 9, 388–394 (2006).
Rogerson, S. J., Hviid, L., Duffy, P. E., Leke, R. F. & Taylor, D. W. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect. Dis. 7, 105–117 (2007).
Langhorne, J., Ndungu, F. M., Sponaas, A. M. & Marsh, K. Immunity to malaria: more questions than answers. Nature Immunol. 9, 725–732 (2008).
Fidock, D. A. et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell 6, 861–871 (2000).
Djimde, A. et al. A molecular marker for chloroquine-resistant falciparum malaria. N. Engl. J. Med. 344, 257–263 (2001).
Laufer, M. K. et al. Return of chloroquine antimalarial efficacy in Malawi. N. Engl. J. Med. 355, 1959–1966 (2006). Demonstrates that the strict removal of chloroquine from Malawi resulted in a decrease in the prevalence of drug-resistant parasites, suggesting that there is a fitness cost associated with chloroquine resistance.
Mita, T. et al. Expansion of wild type allele rather than back mutation in pfcrt explains the recent recovery of chloroquine sensitivity of Plasmodium falciparum in Malawi. Mol. Biochem. Parasitol. 135, 159–163 (2004).
Ginsburg, H. Should chloroquine be laid to rest? Acta Trop. 96, 16–23 (2005).
Sowunmi, A., Adedeji, A. A., Gbotosho, G. O., Fateye, B. A. & Happi, T. C. Effects of pyrimethamine-sulphadoxine, chloroquine plus chlorpheniramine, and amodiaquine plus pyrimethamine-sulphadoxine on gametocytes during and after treatment of acute, uncomplicated malaria in children. Mem. Inst. Oswaldo Cruz 101, 887–893 (2006).
Ogungbamigbe, T., Ojurongbe, O., Ogunro, P., Okanlawon, B. & Kolawole, S. Chloroquine resistant Plasmodium falciparum malaria in Osogbo Nigeria: efficacy of amodiaquine + sulfadoxine-pyrimethamine and chloroquine + chlorpheniramine for treatment. Mem. Inst. Oswaldo Cruz 103, 79–84 (2008).
Sadasivaiah, S., Tozan, Y. & Breman, J. G. Dichlorodiphenyltrichloroethane (DDT) for indoor residual spraying in Africa: how can it be used for malaria control? Am. J. Trop. Med. Hyg. 77, 249–263 (2007).
Guyatt, H. L. & Snow, R. W. Malaria in pregnancy as an indirect cause of infant mortality in sub-Saharan Africa. Trans. R. Soc. Trop. Med. Hyg. 95, 569–576 (2001).
Steketee, R. W., Nahlen, B. L., Parise, M. E. & Menendez, C. The burden of malaria in pregnancy in malaria-endemic areas. Am. J. Trop. Med. Hyg. 64, 28–35 (2001).
ter Kuile, F. O., van Eijk, A. M. & Filler, S. J. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA 297, 2603–2616 (2007).
Schellenberg, D. et al. Intermittent treatment for malaria and anaemia control at time of routine vaccinations in Tanzanian infants: a randomised, placebo-controlled trial. Lancet 357, 1471–1477 (2001).
Macete, E. et al. Intermittent preventive treatment for malaria control administered at the time of routine vaccinations in Mozambican infants: a randomized, placebo-controlled trial. J. Infect. Dis. 194, 276–285 (2006).
Kobbe, R. et al. Malaria incidence and efficacy of intermittent preventive treatment in infants (IPTi). Malar. J. 6, 163 (2007).
White, N. J. How antimalarial drug resistance affects post-treatment prophylaxis. Malar. J. 7, 9 (2008).
UNICEF. Malaria and children: progress in intervention coverage (UNICEF, New York, 2007).
Acknowledgements
We thank I. Borghini-Fuhrer and C. Li for their critical reading of the manuscript. R.T.E. is supported in part by the Training Program in Microbiology for Infectious Diseases (T32 AI007161, Department of Microbiology & Immunology, Columbia University Health Sciences, New York, USA). Funding for this work was also provided in part by the National Institute of Allergy and Infectious Diseases (R01 AI079709). We also thank T. Harris (Graphic Arts Center, Albert Einstein College of Medicine, Bronx, New York) for her initial input into developing figure 2. Our thanks extend also to A. Guilloux (WHO, Geneva) and P. Salama and E. White Johansson (UNICEF, New York) for providing the information for figures 1 and 3.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
41579_2009_BFnrmicro2239_MOESM1_ESM.pdf
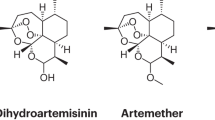
Supplementary information S1 (figure)| Structures of artemisinin derivatives and partner drugs that comprise the most commonly used artemisinin-based combination therapies (PDF 88 kb)
Related links
Related links
DATABASES
Entrez Genome Project
FURTHER INFORMATION
Affordable Medicines Facility – malaria
Bill and Melinda Gates Foundation
The Global Fund to Fight AIDS, Tuberculosis and Malaria
Glossary
- Artemisinin-based combination therapy
-
A combination of artemisinin or one of its derivatives with one or more antimalarials of a different chemical class.
- Pharmacokinetic properties
-
Characteristics of a drug, including its mechanisms of absorption and distribution, the rate at which a drug action begins and the duration of the effect, the chemical changes of the agent in the body, and the effects and routes of excretion of drug metabolites.
- Antimalarial resistance
-
The ability of a parasite strain to survive and multiply despite the administration and adsorption of a drug given in doses equal to or higher than those usually recommended but within tolerance of the subject. The form of the drug that is active against the parasite must be able to gain access to the parasite or to the infected red blood cell for the duration that is necessary for its normal action.
- Recrudescence
-
The reappearance of asexual parasitaemia, after initial parasite clearance, that results from the same infection that caused the original illness.
- Pharmacodynamic properties
-
These include: the physiological effects of a drug on the body, on microorganisms or on parasites in or on the body; the mechanisms of drug action; and the relationship between drug concentration and effect. Pharmacodynamics is often summarized as the study of what a drug does to the body, whereas pharmacokinetics is the study of what the body does to a drug.
- Gametocyte
-
A sexual form of the intra-erythrocytic Plasmodium parasite that matures over a 2-week period, after which it can transmit to Anopheles mosquito vectors. Following ingestion during the insect blood meal, a gametocyte transforms rapidly into a female or male gamete that can undergo sexual recombination in the mosquito midgut.
- Asexual blood-stage trophozoite
-
An asexual form of the intra-erythrocytic Plasmodium parasite that is undergoing cell growth and nuclear division, in preparation for parasite differentiation into a mature schizont that produces individual progeny (known as merozoites). These merozoites burst from the infected cell, ready to initiate new rounds of intracellular development.
- Selection pressure
-
Evolutionary pressure that allows certain genotypes to outcompete others. In the case of malaria, resistance to antimalarials disseminates owing to the selective survival advantage that resistant parasites have in the presence of the drug. In a given population, the greater the proportion of parasites that are exposed to antimalarials at concentrations that allow proliferation only of resistant parasites, the greater the selection pressure.
- Pharmacovigilance
-
The pharmacological science relating to the detection, assessment, understanding and prevention of adverse effects resulting from the short- or long-term use of medicines.
Rights and permissions
About this article
Cite this article
Eastman, R., Fidock, D. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat Rev Microbiol 7, 864–874 (2009). https://doi.org/10.1038/nrmicro2239
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2239
This article is cited by
-
Development of solid lipid nanoparticles-loaded drugs in parasitic diseases
Discover Nano (2024)
-
Trio fluorophore-based phenotypic assay for the detection of artemisinin-induced growth-arrested Plasmodium falciparum in human erythrocytes
Scientific Reports (2024)
-
Design, Synthesis, Molecular Docking, Drug-Likeness/ADMET and Molecular Dynamics Studies of Thiazolyl Benzenesulfonamide Carboxylates as Antimalarial Agents
Chemistry Africa (2024)
-
CHSY1 promotes CD8+ T cell exhaustion through activation of succinate metabolism pathway leading to colorectal cancer liver metastasis based on CRISPR/Cas9 screening
Journal of Experimental & Clinical Cancer Research (2023)
-
Plasmodium falciparum drug resistance-associated mutations in isolates from children living in endemic areas of Burkina Faso
Malaria Journal (2023)