Key Points
-
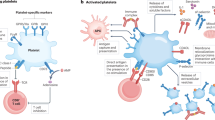
Platelets have multiple direct and indirect functions that integrate innate and adaptive antimicrobial host defences.
-
Platelets actively sense signals of tissue injury and microbial infection via an array of constitutive and inducible membrane receptors.
-
Activated platelets deliver antimicrobial effector molecules, such as kinocidins and platelet microbicidal proteins, to sites of tissue injury or infection and in the bloodstream.
-
Platelet products inhibit viral, bacterial, fungal and protozoan pathogens; for example, new studies demonstrate the importance of kinocidins in human defence against malaria and HIV-1.
-
Platelet interactions with T cells, B cells, neutrophils and other immune system components connect innate and adaptive immunity to promote optimal host defence against infection.
-
Thrombocytopenia and platelet antagonists that inhibit the antimicrobial roles of platelets may be risk factors for infection.
Abstract
Platelets have traditionally been viewed as fragmentary mediators of coagulation. However, recent molecular and cellular evidence suggests that they have multiple roles in host defence against infection. From first-responders that detect pathogens and rapidly deploy host-defence peptides, to beacons that recruit and enhance leukocyte functions in the context of infection, to liaisons that facilitate the T cell–B cell crosstalk that is required in adaptive immunity, platelets represent a nexus at the intersection of haemostasis and antimicrobial host defence. In this Review, I consider recent insights into the antimicrobial roles of platelets, which are mediated both directly and indirectly to integrate innate and adaptive immune responses to pathogens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Luo, D. et al. Protective roles for fibrin, tissue factor, plasminogen activator inhibitor-1, and thrombin activatable fibrinolysis inhibitor, but not factor XI, during defense against the Gram-negative bacterium Yersinia enterocolitica. J. Immunol. 187, 1866–1876 (2011).
Drake, T. A. & Pang, M. Staphylococcus aureus induces tissue factor expression in cultured human cardiac valve endothelium. J. Infect. Dis. 157, 749–756 (1988).
Al Dieri, R., de Laat, B. & Hemker, H. C. Thrombin generation: what have we learned? Blood Rev. 26, 197–203 (2012).
Colman, R. W. Receptors that activate platelets. Proc. Soc. Exp. Biol. Med. 197, 242–248 (1991).
Yeaman, M. R. The role of platelets in antimicrobial host defense. Clin. Infect. Dis. 25, 951–968 (1997).
Yeaman, M. R. Bacterial–platelet interactions: virulence meets host defense. Future Microbiol. 5, 471–506 (2010).
Yeaman, M. R. & Bayer, A. S. in Platelets 3rd edn (ed. Michelson, A.) 767–801 (Academic Press, 2013).
Yeaman, M. R. Platelets in defense against bacterial pathogens. Cell. Mol. Life Sci. 67, 525–544 (2010).
Price, B. & Flaumenhaft, R. Platelet α-granules: basic biology and clinical correlates. Blood Rev. 23, 177–189 (2009).
Fitzgerald, J. R., Foster, T. J. & Cox, D. The interaction of bacterial pathogens with platelets. Nature Rev. Microbiol. 4, 445–457 (2006). An excellent review of platelet interactions from the perspective of the bacterium.
Elzey, B. D., Ratliff, T. L., Sowa, J. M. & Crist, S. A. Platelet CD40L at the interface of adaptive immunity. Thromb. Res. 127, 180–183 (2011).
Engelmann, B. & Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nature Rev. Immunol. 13, 34–45 (2013).
Levin, J. in Platelets 3rd edn (ed. Michelson, A.) 3–25 (Academic Press, 2013).
Weyrich, A. S., Schwertz, H., Kraiss, L. W. & Zimmerman, G. A. Protein synthesis by platelets: historical and new perspectives. J. Thromb. Haemost. 7, 241–246 (2009).
Jenne, C. N., Urrutia, R. & Kubes, P. Platelets: bridging hemostasis, inflammation, and immunity. Int. J. Lab. Hematol. 35, 254–261 (2013).
Youssefian, T., Drouin, A., Masse, J. M., Guichard, J. & Cramer, E. M. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood 99, 4021–4029 (2002).
Zielinski, T., Wachowicz, B., Saluk-Juszczak, J. & Kaca, W. The generation of superoxide anion in blood platelets in response to different forms of Proteus mirabilis lipopolysaccharide: effects of staurosporin, wortmannin, and indomethacin. Thromb. Res. 103, 149–155 (2001).
Lopes-Pires, M. E. et al. Lipopolysaccharide treatment reduces rat platelet aggregation independent of intracellular reactive-oxygen species generation. Platelets 23, 195–201 (2012).
Zarbock, A., Polanowska-Grabowska, R. K. & Ley, K. Platelet–neutrophil-interactions: linking hemostasis and inflammation. Blood Rev. 21, 99–111 (2007).
Baldwin, W. M. 3rd, Kuo, H. H. & Morrell, C. N. Platelets: versatile modifiers of innate and adaptive immune responses to transplants. Curr. Opin. Organ Transplant. 16, 41–46 (2011).
Bielecki, T., Dohan Ehrenfest, D. M., Everts, P. A. & Wiczkowski, A. The role of leukocytes from L-PRP/L-PRF in wound healing and immune defense: new perspectives. Curr. Pharm. Biotechnol. 13, 1153–1162 (2012).
Cerletti, C., Tamburrelli, C., Izzi, B., Gianfagna, F. & de Gaetano, G. Platelet–leukocyte interactions in thrombosis. Thromb. Res. 129, 263–266 (2012).
Li, C. et al. Crosstalk between platelets and the immune system: old systems with new discoveries. Adv. Hematol. 2012, 384685 (2012).
Projahn, D. & Koenen, R. R. Platelets: key players in vascular inflammation. J. Leukoc. Biol. 92, 1167–1175 (2012).
Semple, J. W., Italiano, J. E. Jr, Freedman, J. Platelets and the immune continuum. Nature Rev. Immunol. 11, 264–274 (2011).
Kaushansky, K. Historical review: megakaryopoiesis and thrombopoiesis. Blood 111, 981–986 (2008).
Italiano, J. E. & Shivdasani, R. A. Megakaryocytes and beyond: the birth of platelets. J. Thromb. Haemo. 1, 1174–1182 (2003).
Yeaman, M. R. & Yount, N. Y. Unifying themes in host defence effector polypeptides. Nature Rev. Microbiol. 5, 727–740 (2007). This review provides a synthesis of structural, mechanistic and evolutionary insights regarding host-defence polypeptides.
Yount, N. Y. & Yeaman, M. R. Peptide antimicrobials: cell wall as a bacterial target. Ann. NY Acad. Sci. 1277, 127–138 (2013).
Cox, D., Kerrigan, S. W. & Watson, S. P. Platelets and the innate immune system: mechanisms of bacterial-induced platelet activation. J. Thromb. Haemost. 9, 1097–1107 (2011).
Yount, N. Y. & Yeaman, M. R. Emerging themes and therapeutic prospects for anti-infective peptides. Annu. Rev. Pharmacol. Toxicol. 52, 337–360 (2012).
Metcalf-Pate, K. A. et al. Platelet activation and platelet–monocyte aggregate formation contribute to decreased platelet count during acute simian immunodeficiency virus infection in pig-tailed macaques. J. Infect. Dis. 208, 874–883 (2013).
Gardiner, E. E. & Andrews, R. K. Platelets: envoys at the infection frontline. J. Infect. Dis. 208, 871–873 (2013).
Del Conde, I., Crúz, M. A., Zhang, H., López, J. A. & Afshar-Kharghan, V. Platelet activation leads to activation and propagation of the complement system. J. Exp. Med. 201, 871–879 (2005).
Czapiga, M., Gao, J. L., Kirk, A. & Lekstrom-Himes, J. Human platelets exhibit chemotaxis using functional N-formyl peptide receptors. Exp. Hematol. 33, 73–84 (2005).
Clemetson, K. et al. Functional expression of CCR1, CCR3, CCR4, and CXCR4 chemokine receptors on human platelets. Blood 96, 4046–4054 (2000).
Lowenhaupt, R. W. Human platelet chemotaxis: requirement for plasma factor(s) and the role of collagen. Am. J. Physiol. 235, H23–H28 (1978). This paper provides early evidence for vectorial chemotaxis by human platelets.
Lowenhaupt, R. W., Silberstein, E. B., Sperling, M. I. & Mayfield, G. A quantitative method to measure human platelet chemotaxis using indium-111-oxine-labeled gel-filtered platelets. Blood 60, 1345–1352 (1982).
López, J. A. The platelet Fc receptor: a new role for an old actor. Blood 121, 1674–1675 (2013).
Daga, S. et al. Platelet receptor polymorphisms do not influence Staphylococcus aureus–platelet interactions or infective endocarditis. Microbes Infect. 13, 216–225 (2011).
Qian, K. et al. Functional expression of IgA receptor FcαRI on human platelets. J. Leukoc. Biol. 84, 1492–1500 (2008).
Rogala, B., Gumprecht, J., Gawlik, R. & Strojek, K. Platelet aggregation in IgE-mediated allergy with elevated soluble Fc epsilon RII/CD23 level. J. Investig. Allergol. Clin. Immunol. 5, 161–165 (1995).
Skoglund, C. et al. C-reactive protein and C1q regulate platelet adhesion and activation on adsorbed immunoglobulin G and albumin. Immunol. Cell Biol. 86, 466–474 (2008).
Arman, M., Adams, Y., Lindergard, G. & Rowe, J. A. A method for positive and negative selection of Plasmodium falciparum platelet-mediated clumping parasites and investigation of the role of CD36. PLoS ONE 8, e55453 (2013).
Lord, M. S. et al. The modulation of platelet and endothelial cell adhesion to vascular graft materials by perlecan. Biomaterials. 30, 4898–4906 (2009).
Li, H., Hamza, T., Tidwell, J. E., Clovis, N. & Li, B. Unique antimicrobial effects of platelet-rich plasma and its efficacy as a prophylaxis to prevent implant-associated spinal infection. Adv. Healthc. Mater. 2, 1277–1284 (2013).
Serraino, G. F. et al. Platelet-rich plasma inside the sternotomy wound reduces the incidence of sternal wound infections. Int. Wound J. http://dx.doi.org/10.1111/iwj.12087 (2013).
Martinez-Sapata, M. J. et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst. Rev. 10, CD006899 (2012).
Zhang, G. et al. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J. Immunol. 182, 7997–8004 (2009).
Scott, T. & Owens, M. D. Thrombocytes respond to lipopolysaccharide through Toll-like receptor-4, and MAP kinase and NF-κB pathways leading to expression of interleukin-6 and cyclooxygenase-2 with production of prostaglandin E2. Mol. Immunol. 45, 1001–1008 (2008).
Cognasse, F. et al. Evidence of Toll-like receptor molecules on human platelets. Immunol. Cell Biol. 83, 196–198 (2005).
Rex, S. et al. Immune versus thrombotic stimulation of platelets differentially regulates signalling pathways, intracellular protein–protein interactions, and α-granule release. Thromb. Haemost. 102, 97–110 (2009).
Aslam, R. et al. Platelet toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-α production in vivo. Blood 107, 637–641 (2006).
Shiraki, R. et al. Expression of Toll-like receptors on human platelets. Thromb. Res. 113, 379–385 (2004).
Pancré, V., Monte, D., Delanoye, A., Capron, A. & Aurialt C. Interleukin-6, the main mediator of interaction between monocytes and platelets in killing of Schistosoma mansoni. Eur. Cytokine Net. 1, 15–19 (1990).
Blair, P. et al. Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ. Res. 104, 346–354 (2009).
Assinger, A., Laky, M., Badrnya, S., Esfandeyari, A. & Volf, I. Periodontopathogens induce expression of CD40L on human platelets via TLR2 and TLR4. Thromb. Res. 130, e73–e78 (2012).
Clark, S. R. et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nature Med. 13, 463–469 (2007). This study offers new insights into platelet interactions with neutrophils in host defence.
Cognasse, F. et al. Toll-like receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br. J. Haematol. 141, 84–91 (2008).
Berthet, J. et al. Human platelets can discriminate between various bacterial LPS isoforms via TLR4 signaling and differential cytokine secretion. Clin. Immunol. 145, 189–200 (2012).
Christiansen, D. et al. Differential effect of inhibiting ND-2 and CD14 on LPS- versus whole E. coli bacteria-induced cytokine responses in human blood. Adv. Exp. Med. Biol. 946, 237–251 (2012).
Panigrahi, S. et al. Engagement of platelet toll-like receptor 9 by novel endogenous ligands promotes platelet hyper-reactivity and thrombosis. Circ. Res. 112, 103–112 (2013).
Lachance, C., Segura, M., Gerber, P. P., Xu, J. & Gottschalk, M. Toll-like receptor 2-independent host innate immune response against an epidemic strain of Streptococcus suis that causes a toxic shock-like syndrome in humans. PLoS ONE 8, e65031 (2013).
Dorrington, M. G. et al. MARCO is required for TLR2- and NOD2-mediated responses to Streptococcus pneumoniae and clearance of pneumococcal colonization in the murine nasopharnyx. J. Immunol. 190, 250–258 (2013).
Aktan, I., Dunkel, B. & Cunningham, F. M. Equine platelets inhibit E. coli growth and can be activated by bacterial lipopolysaccharide and lipoteichoic acid although superoxide anion production does not occur and platelet activation is not associated with enhanced production by neutrophils. Vet. Immunol. Immunopath. 152, 209–217 (2013).
Trier, D. et al. Platelet antistaphylococcal responses occur through P2X1 and P2Y12 receptor-induced amplification and kinocidin release. Infect. Immun. 76, 5706–5713 (2008).
Zhang, X. et al. Inhibiting platelet aggregation could aggravate the acute infection caused by Staphylococcus aureus. Platelets 22, 228–236 (2011).
Tilley, D. O. et al. Glycoprotein Iba and FcγRIIa play key roles in platelet activation by the colonizing bacterium, Streptococcus oralis. J. Thromb. Haemost. 11, 941–950 (2013).
Thammavognsa, V., Schneewind, O. & Missiakas, D. M. Enzymatic properties of Staphylococcus aureus adenosine synthase (AdsA). BMC Biochem. 12, 56–61 (2011).
Dürr, M. & Peschel, A. Chemokines meet defensins: the merging concepts of chemoattractants and antimicrobial peptides in host defense. Infect. Immun. 70, 6515–6517 (2002).
Yeaman, M. R. & Yount, N. Y. Code among chaos: immunorelativity and the AEGIS model of antimicrobial peptides. Microbe 71, 21–27 (2005).
Yount, N. Y. & Yeaman, M. R. Structural congruence among membrane-active host defense polypeptides of diverse phylogeny. Biochim. Biophys. Acta 1758, 1373–1386 (2006).
Yount, N. Y. et al. Selective reciprocity in antimicrobial activity versus cytotoxicity of hBD-2 and crotamine. Proc. Natl Acad. Sci. USA 106, 14972–1 4977.
Yount, N. Y. & Yeaman, M. R. Multidimensional signatures in antimicrobial peptides. Proc. Natl Acad. Sci. USA 101, 7363–7368 (2004). This paper reports the discovery of the γ -core motif, which unifies all cysteine-stabilized host-defence peptides.
Yeaman, M. R. et al. Modular determinants of antimicrobial activity in platelet factor-4 family kinocidins. Biochem. Biophys. Acta 1768, 609–619 (2007).
Yount, N. Y. et al. Structural correlates of antimicrobial efficacy in IL-8 and related human kinocidins. Biochim. Biophys. Acta 1768, 598–608 (2007).
Yeaman, M. R., Puentes, S. M., Norman, D. C. & Bayer, A. S. Partial purification and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infect. Immun. 60, 1202–1209 (1992).
Tang, Y. Q., Yeaman, M. R. & Selsted, M. E. Purification, characterization, and antimicrobial properties of peptides released from thrombin-induced human platelets. Blood 86, 910a (1995).
Yeaman, M. R., Tang, Y.-Q., Shen, A. J., Bayer, A. S. & Selsted, M. E. Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect. Immun. 63, 1023–1031 (1997).
Tang, Y. Q., Yeaman, M. R. & Selsted, M. E. Antimicrobial peptides from human platelets. Infect. Immun. 70, 6524–6533 (2002).
Dankert, J., Krijgsveld, J., van Der Werff, J., Joldersma, W. & Zaat, S. A. Platelet microbicidal activity is an important defense factor against viridans streptococcal endocarditis. J. Infect. Dis. 184, 597–605 (2001). This paper makes the important observation that platelets have a role in antimicrobial defence.
Yount, N. Y. et al. Platelet microbicidal protein-1: structural themes of a multifunctional antimicrobial peptide. Antimicrob. Agents Chemother. 48, 4395–4404 (2004).
Schaffner, A., Rhyn, P., Schoedon, G. & Schaer, D. Regulated expression of platelet factor 4 in human monocytes — role of PARs as a quantitatively important monocyte activation pathway. J. Leuk. Biol. 78, 202–209 (2005).
Yount, N. Y., Andrés, M. T., Fierro, J. F. & Yeaman, M. R. The γ-core motif correlates with antimicrobial activity in Cys-containing kaliocin-1 originating from transferrins. Biochem. Biophys. Acta 1768, 2862–2872 (2007).
Krijgsveld, J. et al. Thrombocidins, microbicidal proteins from human blood platelets, are C-terminal deletion products of CXC chemokines. J. Biol. Chem. 275, 20374–20381 (2000).
Bourbigot, S. et al. Antimicrobial peptide RP-1 structure and interactions with anionic versus zwitterionic micelles. Biopolymers 91, 1–13 (2009).
Bourbigot, S., Fardy, L., Waring, A., Yeaman, M. R. & Booth, V. Structure of chemokine-derived antimicrobial peptide IL-8α and interaction with detergent micelles and oriented lipid bilayers. Biochemistry 48, 10509–10521 (2009).
Yount, N. Y. et al. Context mediates antimicrobial efficacy of kinocidin congener peptide RP-1. PLoS ONE 6, e26727 (2011).
Solomon Tsegaye, T. et al. Platelet activation suppresses HIV-1 infection of T cells. Retrovirology 10, http://dx.doi.org/10.1186/1742-4690-10-48 (2013).
Cocchi, F. et al. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science 270, 1811–1815 (1995).
Cocchi, F. et al. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nature Med. 2, 1244–1247 (1996).
Burns, J. M., Gallo, R. C., DeVico, A. L. & Lewis, G. K. A new monoclonal antibody, mAb 4A12, identifies a role for the glycosaminoglycan (GAG) binding domain of RANTES in the antiviral effect against HIV-1 and intracellular calcium Ca2+ signaling. J. Ex. Med. 188, 1917–1927 (1998).
Auerbach, D. J. et al. Identification of the platelet-derived chemokine CXCL4/PF4 as a broad-spectrum HIV-inhibitor. Proc. Natl Acad. Sci. USA 109, 9569–9574 (2012).
Mercier, R. C., Dietz, R. M., Mazzola, J. L., Bayer, A. S. & Yeaman, M. R. Beneficial influence of platelets on antibiotic efficacy in an in vitro model of Staphylococcus aureus induced endocarditis. Antimicrob. Agents Chemother. 48, 2551–2557 (2004).
Sullam, P. M. et al. Effect of thrombocytopenia on the early course of streptococcal endocarditis. J. Infect. Dis. 168, 910–914 (1993). This paper is among the first studies to demonstrate the antimicrobial function of platelets in vivo.
Wong, C. H. Y., Jenne, C. N., Petri, B., Chrobok, N. & Kubes, P. Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nature Immunol. 14, 785–792 (2013).
McMorran, B. J. et al. Platelet factor 4 and Duffy antigen required for platelet killing of Plasmodium falciparum. Science 338, 1348–1351 (2012). This paper represents a major advance in our understanding of host defence against malaria.
Love, M. S. et al. Platelet factor 4 activity against P. falciparum and its translation to nonpeptidic mimics as antimalarials. Cell Host Microbe 12, 815–823 (2012).
Wilson, N. O. et al. Pharmacologic inhibition of CXCL10 in combination with anti-malarial therapy eliminates mortality associated with murine model of cerebral malaria. PLoS ONE 8, e60898 (2013).
Rieg,G. et al. Platelet count is associated with plasma HIV type 1 RNA and disease progression. AIDS Res. Hum. Retroviruses 23, 1257–1261 (2007).
Coelho, H. C. et al. Thrombocytopenia in Plasmodium vivax malaria is related to platelet phagocytosis. PLoS ONE 8, e63410 (2013).
Yeaman, M. R. et al. Resistance to platelet microbicidal protein results in increased severity of experimental Candida albicans endocarditis. Infect. Immun. 64, 1379–1384 (1996).
Perkhofer, S. et al. Human platelets attenuate Aspergillus species via granule-dependent mechanisms. J. Infect. Dis. 198, 1243–1246 (2008).
Christin, L. et al. Human platelets damage Aspergillus fumigatus hyphae and may supplement killing by neutrophils. Infect. Immun. 66, 1181–1189 (1998). This paper provides mechanistic evidence for the anti-aspergillus functions of platelets ex vivo.
Chumpitazi, B. F. et al. Human platelet inhibition of Toxoplasma gondii growth. Clin. Exp. Immunol. 111, 325–333 (1998).
Erfe, M. C. et al. Efficacy of synthetic peptides RP-1 and AA-RP-1 against Leishmania species in vitro and in vivo. Antimicrob. Agents Chemother. 56, 658–665 (2012).
Stanley, R. G., Ngaiza, J. R., Wambayi, E., Lewis, J. & Doenhoff, M. J. Platelets as an innate defence mechanism against Schistosoma mansoni infections in mice. Parasite Immunol. 25, 467–473 (2003).
Dorit, M., Zander, W. & Klinger, M. H. F. The blood platelets contribution to innate host defense — what they have learned from their big brothers. Biotechnol. J. 4, 914–926 (2009).
Joseph, M. et al. IgE-dependent platelet cytotoxicity against helminths. Adv. Exp. Med. Biol. 184, 23–33 (1985).
White, J. G. Platelets are covercytes, not phagocytes: uptake of bacteria involves channels of the open canalicular system. Platelets 16, 121–131 (2005).
Stephen, J., Emerson, B., Fox, K. A. & Dransfield, I. The uncoupling of monocyte–platelet interactions from the induction of proinflammatory signaling in monocytes. J. Immunol. 191, 5677–5683 (2013).
Johansson, D., Shannon, O. & Rasmussen, M. Platelet and neutrophil responses to Gram-positive pathogens in patients with bacteremic infection. PLoS ONE 6, e26928 (2011).
McDonald, B., Urrutia, R., Yipp, B. G., Jenne, C. N. & Kubes, P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 12, 324–333 (2012).
Jenne, C. N. et al. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe 13, 169–180 (2013).
Miedzobrodzki, J. et al. Platelets augment respiratory burst in neutrophils activated by selected species of gram-positive or Gram-negative bacteria. Folia Histochem. Cytobiol. 46, 383–388 (2008).
Yeaman, M. R. Host defense in the endovascular compartment. Emerging concepts in endovascular infection symposium (032B), Abstract 326 (American Society for Microbiology, 51st ICAAC, 2011).
Maier, M. et al. Platelet factor 4 is highly upregulated in dendritic cells after severe trauma. Mol. Med. 15, 384–391 (2009).
Verschoor, A. et al. A platelet-mediated system for shuttling blood-borne bacteria to CD8α+ dendritic cells depends on glycoprotein GPIb and complement C3. Nature Immunol. 12, 1194–1201 (2011). This paper offers key new insights into the role of platelets in antigen presentation and processing.
Nguyen, X. D. et al. Differentiation of monocyte-derived dendritic cells under the influence of platelets. Cytotherapy 10, 720–729 (2008).
Antczak, A. J., Vieth, J. A., Singh, N. & Worth, R. G. Internalization of IgG-coated targets results in activation and secretion of soluble CD40 ligand and RANTES by human platelets. Clin. Vaccine Immunol. 18, 210–216 (2011).
Elzey, B. D. et al. Platelet-derived CD154 enables T-cell priming and protection against Listeria monocytogenes challenge. Blood 111, 3684–3691 (2008).
Sowa, J. M., Crist, S. A., Ratliff, T. L. & Elzey, B. D. Platelet influence on T- and B-cell responses. Arch. Immunol. Ther. Exp. 57, 235–241 (2009).
Iannacone, M. et al. Platelets prevent IFN-α/β-induced lethal hemorrhage promoting CTL-dependent clearance of lymphocytic choriomeningitis virus. Proc. Natl Acad. Sci. USA 105, 629–634 (2008).
Iannacone, M. et al. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nature Med. 11, 1161–1169 (2005).
Gerdes, N. et al. Platelets regulate CD4+ T-cell differentiation via multiple chemokines in humans. Thromb. Haemost. 106, 353–362 (2011).
Maione, F. et al. IL-17A increases ADP-induced platelet aggregation. Biochem. Biophys. Res. Commun. 408, 658–662 (2011).
Zhang, S. et al. IL-17A facilitates platelet function through the ERK2 signaling pathway in patients with acute coronary syndrome. PLoS ONE 7, e40641 (2012).
Liu, C. Y. et al. Platelet factor 4 differentially modulates CD4+CD25+ (regulatory) versus CD4+CD25− (non-regulatory) T cells. J. Immunol. 174, 2680–2686 (2005).
Elzey, B. D. et al. Cooperation between platelet-derived CD154 and CD4+ T cells for enhanced germinal center formation. J. Leukoc. Biol. 78, 80–84 (2005).
Elzey, B. D., Sprague, D. L. & Ratliff, T. L. The emerging role of platelets in adaptive immunity. Cell. Immunol. 238, 1–9 (2005). This is an important review of the functions of platelets in adaptive immunity.
Martinson, J., Bae, J., Klingemann, H. G. & Tam, Y. Activated platelets rapidly up-regulate CD40L expression and can effectively mature and activate autologous ex vivo differentiated DC. Cytotherapy 6, 487–497 (2004).
Elzey, B. D. et al. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity 19, 9–19 (2003).
Sprague, D. L. et al. Platelet-mediated modulation of adaptive immunity: unique delivery of CD154 signal by platelet-derived membrane vesicles. Blood 11, 5028–5036 (2008).
Ngyuen, T., Ghebrehiwet, B. & Peerschke, E. Staphylococcus aureus protein A recognizes platelet gC1qR/p33: a novel mechanism for staphylococcal interactions with platelets. Infect. Immun. 68, 2061–2068 (2000).
Kerrigan, S. W. et al. Role of Streptococcus gordonii surface proteins SspA/SspB and Hsa in platelet function. Infect. Immun. 75, 5740–5747 (2007).
Mishra, N. N. et al. Emergence of daptomycin resistance in daptomycin-naive rabbits with methicillin-resistant Staphylococcus aureus prosthetic joint infection is associated with resistance to host defense cationic peptides and mprF polymorphisms. PLoS ONE 8, e71151 (2013).
Jones, T. et al. Failures in clinical treatment of Staphylococcus aureus infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob. Agents Chemother. 52, 269–278 (2008).
Witjen, P. et al. High precision platelet releasate definition by quantitative reversed protein profilining — brief report. Arterioscl. Throm. Vas. Biol. 33, 1635–1638 (2013).
Ivanov, I. B., Gritsenko, V. A. & Kuzmin, M. D. Staphylococcal secretory inhibitor of platlelet microbicidal protein is associated with prostatitis source. J. Med. Microbiol. 55, 1645–1648 (2006).
Kahn, F., Hurley, S. & Shannon, O. Platelets promote bacterial dissemination in a mouse model of streptococcal sepsis. Microbes Infect. 15, 669–676 (2013).
Lo, E. & Deane, S. Diagnosis and classification of immune-mediated thrombocytopenia. Autoimmun. Rev. 13, 577–583 (2014). [Au:OK?]
Yoon, J. H. et al. Liver abscess due to Klebsiella pneumonia: risk factors for metastatic infection. Scand. J. Infect. Dis. 46, 21–26 (2014).
Wang, J. T., Wu, H. S., Weng, C. M., Hsu, L. Y. & Wang, F. D. Prognosis of patients with methicillin-resistant Staphylococcus aureus bloodstream infection treated with teichoplanin: a retrospective cohort study investigating effect of teichoplanin minimum inhibitory concentrations. BMC Infect. Dis. 13, 182–186 (2013).
Chang, F. Y. et al. Thrombocytopenia in liver transplant recipients: predictors, impact on fungal infections, and role of endogenous thrombopoietin. Transplantation 69, 70–75 (2000).
Kupferwasser, L. I. et al. Salicylic acid attenuates virulence in endovascular infections by targeting global regulatory pathways in Staphylococcus aureus. J. Clin. Invest. 112, 222–133 (2003).
Kupferwasser, L. I. et al. Acetylsalicylic acid reduces vegetation bacterial density, hematogenous bacterial dissemination, and frequency of embolic events in experimental Staphylococcus aureus endocarditis through antiplatelet and antibacterial effects. Circulation 99, 2791–2797 (1999). This paper provides novel insights into the antimicrobial mechanisms of platelets and salicylates.
Nicolau, D. P., Tessier, P. R. & Nightingale, C. H. Beneficial effect of combination antiplatelet therapy on the development of experimental Staphylococcus aureus endocarditis. Int. J. Antimicrob. Agents 11, 159–161 (1999).
Nicolau, D. P., Tessier, P. R., Nightingale, C. H. & Quintiliani, R. Influence of adjunctive ticlopidine on the treatment of experimental Staphylococcus aureus endocarditis. Int. J. Antimicrob. Agents 9, 227–229 (1998).
Nicolau, D. P., Marangos, M. N., Nightingale, C. H. & Quintiliani, R. Influence of aspirin on development and treatment of experimental Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 39, 1748–1751 (1995). This paper makes important observations regarding the efficacy of aspirin in experimental infections.
Park, M. H. et al. Modulation of regulated virulence gene expression within MRSA using salicylate analogues. Am. Soc. Microb. Abstr. B-3569 (2008).
Sedlacek, M., Gemery, J. M., Cheung, A. L., Bayer, A. S. & Remillard, B. D. Aspirin treatment is associated with a significantly decreased risk of Staphylococcus aureus bacteremia in hemodialysis patients with tunneled catheters. Am. J. Kidney Dis. 49, 401–408 (2007).
Eisen, D. P. et al. Reduced valve replacement surgery and complication rate in Staphylococcus endocarditis patients receiving acetylsalicylic acid. J. Infect. 58, 332–338 (2009).
Habib, A., Baddour, L. & Sohail-Rizwan, M. Impact of anti-platelet therapy on clinical manifestations and outcomes of cardiovascular infection. Curr. Infect. Dis. Rep. 15, 347–352 (2013).
Chan, K. L. et al. A randomized trial of aspirin on the risk of embolic events in patients with infective endocarditis. J. Am. Coll. Cardiol. 42, 775–780 (2003).
Acknowledgements
This Review highlights recent advances in platelet immunology, focusing on antimicrobial host defence. This field of research has been the area of many important efforts on the part of numerous excellent investigators over many years of work. Although only a few studies could be illustrated in the scope of this Review, appreciation is extended to all those who work in this important area. M.R.Y. was supported, in part, by R01 grants AI39001 and AI48031 from the US National Institutes of Health and grant W81XWH-12-2-0101 from the US Department of Defense.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M.R.Y. is a founder and shareholder of NovaDigm Therapeutics, Inc., and founder and shareholder of Metacin, Inc., neither of which provided support for the current manuscript. He is a cited inventor of anti-infective peptides and mimetics thereof, as well as of cross-kingdom vaccines.
Rights and permissions
About this article
Cite this article
Yeaman, M. Platelets: at the nexus of antimicrobial defence. Nat Rev Microbiol 12, 426–437 (2014). https://doi.org/10.1038/nrmicro3269
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro3269
This article is cited by
-
Assistive diagnostic indicators for infections related to lumbar posterior interbody fusion internal fixation: platelet count and mean platelet volume
Journal of Orthopaedic Surgery and Research (2023)
-
Platelet-rich plasma in the treatment of anal fistula: a systematic review and meta-analysis
International Journal of Colorectal Disease (2023)
-
Systematic literature review evaluating evidence and mechanisms of action for platelet-rich plasma as an antibacterial agent
Journal of Cardiothoracic Surgery (2021)
-
PI3K/Akt signaling pathway is essential for de novo hair follicle regeneration
Stem Cell Research & Therapy (2020)
-
Patrolling the vascular borders: platelets in immunity to infection and cancer
Nature Reviews Immunology (2019)