Key Points
-
HIV-1 infection will remain an incurable disease until persistent, latent infection of long-lived memory CD4+ T cells is successfully targeted.
-
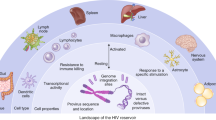
Resting memory CD4+ T cells are the best characterized reservoir of latent HIV-1 infection, but other potential cellular reservoirs of persistent, latent HIV-1 infection, including CD4+ memory stem cells and γδ T cells, have recently been reported. In addition, macrophages may be a source of persistent infection, but whether these cells harbour true latent infection is still unclear.
-
The mechanisms that control HIV-1 latency are multifactorial, and thus it will probably be necessary to combine complementary approaches to eradicate HIV-1 infection.
-
Humanized mouse and non-human primate models of HIV-1 will continue to have an essential role in HIV-1 eradication research. Development of reliable primary cell models of HIV-1 latency will also help in the evaluation of novel approaches.
-
Development of reliable, sensitive and tractable assays that are capable of measuring the in vivo impact of anti-latency compounds and eradication protocols in clinical trial evaluation is a key unmet need.
-
Histone deacetylase inhibitors have emerged as the leading anti-latency candidates to advance into the clinics. However, latency-reactivating agents alone will not be sufficient to clear infection, and strategies to enhance the immune response will also have to be used.
Abstract
Effective antiretroviral therapy (ART) blunts viraemia, which enables HIV-1-infected individuals to control infection and live long, productive lives. However, HIV-1 infection remains incurable owing to the persistence of a viral reservoir that harbours integrated provirus within host cellular DNA. This latent infection is unaffected by ART and hidden from the immune system. Recent studies have focused on the development of therapies to disrupt latency. These efforts unmasked residual viral genomes and highlighted the need to enable the clearance of latently infected cells, perhaps via old and new strategies that improve the HIV-1-specific immune response. In this Review, we explore new approaches to eradicate established HIV-1 infection and avoid the burden of lifelong ART.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Persaud, D. in 20th International AIDS Conference. Symposium presentation MOSY0501 (International AIDS Society, 2014).
Henrich, T. J. et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann. Intern. Med. 161, 319–327 (2014).
Chun, T. W. et al. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nature Med. 1, 1284–1290 (1995).
Chun, T. W. et al. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc. Natl Acad. Sci. USA 95, 8869–8873 (1998).
Strain, M. C. et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J. Infect. Dis. 191, 1410–1418 (2005).
Archin, N. M. et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc. Natl Acad. Sci. USA 109, 9523–9528 (2012).
Swiggard, W. J. et al. Human immunodeficiency virus type 1 can establish latent infection in resting CD4+ T cells in the absence of activating stimuli. J. Virol. 79, 14179–14188 (2005).
Bukrinsky, M. I., Stanwick, T. L., Dempsey, M. P. & Stevenson, M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science 254, 423–427 (1991). This paper identifies resting T lymphocytes as a major reservoir of HIV-1 infection in which the virus can be reactivated following cell stimulation.
Zack, J. A. et al. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61, 213–222 (1990).
Zhou, Y., Zhang, H., Siliciano, J. D. & Siliciano, R. F. Kinetics of human immunodeficiency virus type 1 decay following entry into resting CD4+ T cells. J. Virol. 79, 2199–2210 (2005).
Siliciano, J. D. et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nature Med. 9, 727–728 (2003). This study reports that the HIV-1 reservoir is highly stable and decays very slowly in successfully treated patients receiving ART with stably undetectable plasma HIV-1 RNA.
Calvanese, V., Chavez, L., Laurent, T., Ding, S. & Verdin, E. Dual-color HIV reporters trace a population of latently infected cells and enable their purification. Virology 446, 283–292 (2013).
Hermankova, M. et al. Analysis of human immunodeficiency virus type 1 gene expression in latently infected resting CD4+ T lymphocytes in vivo. J. Virol. 77, 7383–7392 (2003).
Iglesias-Ussel, M., Vandergeeten, C., Marchionni, L., Chomont, N. & Romerio, F. High levels of CD2 expression identify HIV-1 latently infected resting memory CD4+ T cells in virally suppressed subjects. J. Virol. 87, 9148–9158 (2013).
Josefsson, L. et al. The HIV-1 reservoir in eight patients on long-term suppressive antiretroviral therapy is stable with few genetic changes over time. Proc. Natl Acad. Sci. USA 110, 25 (2013).
Kearney, M. F. et al. Lack of detectable HIV-1 molecular evolution during suppressive antiretroviral therapy. PLoS Pathog. 10, e1004010 (2014).
Palmer, S. et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc. Natl Acad. Sci. USA 105, 3879–3884 (2008).
Dornadula, G. et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA 282, 1627–1632 (1999).
Grossman, Z. et al. Ongoing HIV dissemination during HAART. Nature Med. 5, 1099–1104 (1999).
Frenkel, L. M. et al. Multiple viral genetic analyses detect low-level human immunodeficiency virus type 1 replication during effective highly active antiretroviral therapy. J. Virol. 77, 5721–5730 (2003).
Buzon, M. J. et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nature Med. 16, 460–465 (2010).
Sigal, A. et al. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature 477, 95–98 (2011).
Permanyer, M. et al. Antiretroviral agents effectively block HIV replication after cell-to-cell transfer. J. Virol. 86, 8773–8780 (2012).
Agosto, L. M., Zhong, P., Munro, J. & Mothes, W. Highly active antiretroviral therapies are effective against HIV-1 cell-to-cell transmission. PLoS Pathog. 10, e1003982 (2014).
Titanji, B. K., Aasa-Chapman, M., Pillay, D. & Jolly, C. Protease inhibitors effectively block cell-to-cell spread of HIV-1 between T cells. Retrovirology 10, 1742–4690 (2013).
Yukl, S. A. et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. AIDS 24, 2451–2460 (2010).
Vallejo, A. et al. The effect of intensification with raltegravir on the HIV-1 reservoir of latently infected memory CD4 T cells in suppressed patients. AIDS 26, 1885–1894 (2012).
Gandhi, R. T. et al. No effect of raltegravir intensification on viral replication markers in the blood of HIV-1-infected patients receiving antiretroviral therapy. J. Acquir. Immune Def. Syndr. 59, 229–235 (2012).
Fletcher, C. V. et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc. Natl Acad. Sci. USA 111, 2307–2312 (2014).
Anton, P. A. et al. RMP-02/MTN-006: a phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res. Hum. Retroviruses 28, 1412–1421 (2012).
Robbins, B. L., Nelson, S. R. & Fletcher, C. V. A novel ultrasensitive LC–MS/MS assay for quantification of intracellular raltegravir in human cell extracts. J. Pharm. Biomed. Anal. 70, 378–387 (2012).
Kieffer, T. L. et al. Genotypic analysis of HIV-1 drug resistance at the limit of detection: virus production without evolution in treated adults with undetectable HIV loads. J. Infect. Dis. 189, 1452–1465 (2004).
Bailey, J. R. et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J. Virol. 80, 6441–6457 (2006).
Evering, T. H. et al. Absence of HIV-1 evolution in the gut-associated lymphoid tissue from patients on combination antiviral therapy initiated during primary infection. PLoS Pathog. 8, e1002506 (2012).
Chomont, N. et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nature Med. 15, 893–900 (2009).
Bosque, A., Famiglietti, M., Weyrich, A. S., Goulston, C. & Planelles, V. Homeostatic proliferation fails to efficiently reactivate HIV-1 latently infected central memory CD4+ T cells. PLoS Pathog. 7, 6 (2011).
Maldarelli, F. et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345, 179–183 (2014).
Wagner, T. A. et al. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 345, 570–573 (2014). References 35 and 38 show that HIV-1 integrates into genes associated with cellular proliferation, which may contribute to aberrant proliferation of silenced HIV-1 genomes.
Margolis, D. & Bushman, F. HIV/AIDS. Persistence by proliferation? Science 345, 143–144 (2014).
Lewinski, M. K. et al. Genome-wide analysis of chromosomal features repressing human immunodeficiency virus transcription. J. Virol. 79, 6610–6619 (2005).
Shan, L. et al. Influence of host gene transcription level and orientation on HIV-1 latency in a primary-cell model. J. Virol. 85, 5384–5393 (2011).
Sherrill-Mix, S. et al. HIV latency and integration site placement in five cell-based models. Retrovirology 10, 1742–4690 (2013).
Verdin, E., Paras, P. Jr & Van Lint, C. Chromatin disruption in the promoter of human immunodeficiency virus type 1 during transcriptional activation. EMBO J. 12, 3249–3259 (1993).
Keedy, K. S. et al. A limited group of class I histone deacetylases acts to repress human immunodeficiency virus type 1 expression. J. Virol. 83, 4749–4756 (2009).
Friedman, J. et al. Epigenetic silencing of HIV-1 by the histone H3 lysine 27 methyltransferase enhancer of Zeste 2. J. Virol. 85, 9078–9089 (2011).
Mbonye, U. & Karn, J. Transcriptional control of HIV latency: cellular signaling pathways, epigenetics, happenstance and the hope for a cure. Virology 454–455, 328–339 (2014).
du Chene, I. et al. Suv39H1 and HP1γ are responsible for chromatin-mediated HIV-1 transcriptional silencing and post-integration latency. EMBO J. 26, 424–435 (2007).
Herrmann, C. H. & Rice, A. P. Lentivirus Tat proteins specifically associate with a cellular protein kinase, TAK, that hyperphosphorylates the carboxyl-terminal domain of the large subunit of RNA polymerase II: candidate for a Tat cofactor. J. Virol. 69, 1612–1620 (1995).
Wei, P., Garber, M. E., Fang, S. M., Fischer, W. H. & Jones, K. A. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell 92, 451–462 (1998).
Budhiraja, S., Famiglietti, M., Bosque, A., Planelles, V. & Rice, A. P. Cyclin T1 and CDK9 T-loop phosphorylation are downregulated during establishment of HIV-1 latency in primary resting memory CD4+ T cells. J. Virol. 87, 1211–1220 (2013).
Van Lint, C., Bouchat, S. & Marcello, A. HIV-1 transcription and latency: an update. Retrovirology 10, 1742–4690 (2013).
Descours, B. et al. Immune responses driven by protective human leukocyte antigen alleles from long-term nonprogressors are associated with low HIV reservoir in central memory CD4 T cells. Clin. Infect. Dis. 54, 1495–1503 (2012).
Bacchus, C. et al. A single HIV-1 cluster and a skewed immune homeostasis drive the early spread of HIV among resting CD4+ cell subsets within one month post-infection. PLoS ONE 8, e64219 (2013).
Soriano-Sarabia, N., Archin, N. & Margolis, D. in Conference on Retroviruses and Opportunistic Infections. Abstract 46 (2013).
Zhang, J. & Crumpacker, C. S. Hematopoietic stem and progenitor cells in HIV/AIDS and immune reconstitution. Cell Res. 20, 745–747 (2010).
Carter, C. C. et al. HIV-1 utilizes the CXCR4 chemokine receptor to infect multipotent hematopoietic stem and progenitor cells. Cell Host Microbe 9, 223–234 (2011).
Carter, C. C. et al. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nature Med. 16, 446–451 (2010).
Durand, C. M. et al. HIV-1 DNA is detected in bone marrow populations containing CD4+ T cells but is not found in purified CD34+ hematopoietic progenitor cells in most patients on antiretroviral therapy. J. Infect. Dis. 205, 1014–1018 (2012).
Josefsson, L. et al. Hematopoietic precursor cells isolated from patients on long-term suppressive HIV therapy did not contain HIV-1 DNA. J. Infect. Dis. 206, 28–34 (2012).
Wightman, F. et al. Both CD31+ and CD31− naive CD4+ T cells are persistent HIV type 1-infected reservoirs in individuals receiving antiretroviral therapy. J. Infect. Dis. 202, 1738–1748 (2010).
Buzon, M. J. et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nature Med. 20, 139–142 (2014).
Cieri, N. et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 121, 573–584 (2013).
Gattinoni, L. et al. A human memory T cell subset with stem cell-like properties. Nature Med. 17, 1290–1297 (2011).
Soriano-Sarabia, N. et al. Primary MHC-class II+ cells are necessary to promote resting Vδ2 cell expansion in response to (E)-4-hydroxy-3-methyl-but-2-enyl-pyrophosphate and isopentenyl pyrophosphate. J. Immunol. 189, 5212–5222 (2012).
Morita, C. T. et al. Structural features of nonpeptide prenyl pyrophosphates that determine their antigenicity for human γδ T cells. J. Immunol. 167, 36–41 (2001).
Hintz, M. et al. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett. 509, 317–322 (2001).
Poupot, M. & Fournie, J. J. Non-peptide antigens activating human Vγ9/Vδ2 T lymphocytes. Immunol. Lett. 95, 129–138 (2004).
Vavassori, S. et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nature Immunol. 14, 908–916 (2013).
Igarashi, T. et al. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc. Natl Acad. Sci. USA 98, 658–663 (2001).
Gartner, S. et al. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 233, 215–219 (1986).
Koenig, S. et al. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 233, 1089–1093 (1986).
Lambotte, O. et al. Detection of infectious HIV in circulating monocytes from patients on prolonged highly active antiretroviral therapy. J. Acquir. Immune Def. Syndr. 23, 114–119 (2000).
Price, R. W. et al. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science 239, 586–592 (1988).
Gorry, P. R. et al. Diminished production of human immunodeficiency virus type 1 in astrocytes results from inefficient translation of gag, env, and nef mRNAs despite efficient expression of Tat and Rev. J. Virol. 73, 352–361 (1999).
Sieweke, M. H. & Allen, J. E. Beyond stem cells: self-renewal of differentiated macrophages. Science 342, 1242974 (2013). This article reviews recent evidence indicating that, like stem cells, macrophages have self-renewing capabilities.
Queen, S. E. et al. Replication-competent simian immunodeficiency virus (SIV) Gag escape mutations archived in latent reservoirs during antiretroviral treatment of SIV-infected macaques. J. Virol. 85, 9167–9175 (2011). This study determines that SIV Gag escape mutations can be found in the resting CD4+ T cell latent reservoir in the periphery and the CNS.
Denton, P. W. et al. Generation of HIV latency in humanized BLT mice. J. Virol. 86, 630–634 (2012). This study describes the presence and frequency of replication-competent latent HIV-1 in ART-suppressed humanized BLT mice.
Garcia-Martinez, J. in HIV Persistence During Therapy, Sixth International Workshop. Abstract 17 (IHL Press, 2013).
Margolis, D. M. How might we cure HIV? Curr. Infect. Dis. Rep. 16, 392 (2014).
Chun, T. W. et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387, 183–188 (1997).
Yang, H. C. et al. Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J. Clin. Invest. 119, 3473–3486 (2009).
Cameron, P. U. et al. Establishment of HIV-1 latency in resting CD4+ T cells depends on chemokine-induced changes in the actin cytoskeleton. Proc. Natl Acad. Sci. USA 107, 16934–16939 (2010).
Saleh, S. et al. CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection: a novel model of HIV-1 latency. Blood 110, 4161–4164 (2007).
Lassen, K. G., Hebbeler, A. M., Bhattacharyya, D., Lobritz, M. A. & Greene, W. C. A flexible model of HIV-1 latency permitting evaluation of many primary CD4 T-cell reservoirs. PLoS ONE 7, e30176 (2012).
Jordan, A., Bisgrove, D. & Verdin, E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 22, 1868–1877 (2003).
Bosque, A. & Planelles, V. Induction of HIV-1 latency and reactivation in primary memory CD4+ T cells. Blood 113, 58–65 (2009).
Bosque, A. & Planelles, V. Studies of HIV-1 latency in an ex vivo model that uses primary central memory T cells. Methods 53, 54–61 (2011).
Tyagi, M., Pearson, R. J. & Karn, J. Establishment of HIV latency in primary CD4+ cells is due to epigenetic transcriptional silencing and P-TEFb restriction. J. Virol. 84, 6425–6437 (2010).
Spina, C. A. et al. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog. 9, e1003834 (2013). This study compares the responses to different latency-reactivating stimuli in nearly all of the primary cell HIV-1 latency models that have been described to responses obtained using the QVOA in cells from patients infected with HIV-1.
Hakre, S., Chavez, L., Shirakawa, K. & Verdin, E. HIV latency: experimental systems and molecular models. FEMS Microbiol. Rev. 36, 706–716 (2012).
Barton, K. M., Burch, B. D., Soriano-Sarabia, N. & Margolis, D. M. Prospects for treatment of latent HIV. Clin. Pharmacol. Ther. 93, 46–56 (2013).
Shultz, L. D., Ishikawa, F. & Greiner, D. L. Humanized mice in translational biomedical research. Nature Rev. Immunol. 7, 118–130 (2007).
Lan, P., Tonomura, N., Shimizu, A., Wang, S. & Yang, Y. G. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood 108, 487–492 (2006).
Melkus, M. W. et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nature Med. 12, 1316–1322 (2006).
Denton, P. W. et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 5, e16 (2008).
Brainard, D. M. et al. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J. Virol. 83, 7305–7321 (2009).
Denton, P. W. et al. Systemic administration of antiretrovirals prior to exposure prevents rectal and intravenous HIV-1 transmission in humanized BLT mice. PLoS ONE 5, e8829 (2010).
Marsden, M. D. et al. HIV latency in the humanized BLT mouse. J. Virol. 86, 339–347 (2012).
Long, B. R. & Stoddart, C. A. α-interferon and HIV infection cause activation of human T cells in NSG–BLT mice. J. Virol. 86, 3327–3336 (2012).
Zou, W. et al. Nef functions in BLT mice to enhance HIV-1 replication and deplete CD4+CD8+ thymocytes. Retrovirology 9, 44 (2012).
Sun, Z. et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J. Exp. Med. 204, 705–714 (2007).
Denton, P. W. et al. Targeted cytotoxic therapy kills persisting HIV infected cells during ART. PLoS Pathog. 10, e1003872 (2014). In this study, an immunotoxin is evaluated for its capacity to kill HIV-1-expressing cells in a humanized mouse model. The results show a dramatic reduction in HIV-1 RNA in tissue following immunotoxin administration together with ART.
Vatakis, D. N. et al. Using the BLT humanized mouse as a stem cell based gene therapy tumor model. J. Vis. Exp. 70, e4181 (2012).
Shimizu, S. et al. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. Blood 115, 1534–1544 (2010).
Honeycutt, J. B. et al. HIV-1 infection, response to treatment and establishment of viral latency in a novel humanized T cell-only mouse (TOM) model. Retrovirology 10, 121 (2013).
Dinoso, J. B. et al. A simian immunodeficiency virus-infected macaque model to study viral reservoirs that persist during highly active antiretroviral therapy. J. Virol. 83, 9247–9257 (2009). This study shows the frequency and distribution of replication-competent latent SIV in ART-suppressed macaques.
Clements, J. E., Gama, L., Graham, D. R., Mankowski, J. L. & Zink, M. C. A simian immunodeficiency virus macaque model of highly active antiretroviral treatment: viral latency in the periphery and the central nervous system. Curr. Opin. HIV AIDS 6, 37–42 (2011).
Hirsch, V. M., Zack, P. M., Vogel, A. P. & Johnson, P. R. Simian immunodeficiency virus infection of macaques: end-stage disease is characterized by widespread distribution of proviral DNA in tissues. J. Infect. Dis. 163, 976–988 (1991).
Horiike, M. et al. Lymph nodes harbor viral reservoirs that cause rebound of plasma viremia in SIV-infected macaques upon cessation of combined antiretroviral therapy. Virology 423, 107–118 (2012).
Baskin, G. B. et al. Distribution of SIV in lymph nodes of serially sacrificed rhesus monkeys. AIDS Res. Hum. Retroviruses 11, 273–285 (1995).
Shen, A. et al. Resting CD4+ T lymphocytes but not thymocytes provide a latent viral reservoir in a simian immunodeficiency virus–Macaca nemestrina model of human immunodeficiency virus type 1-infected patients on highly active antiretroviral therapy. J. Virol. 77, 4938–4949 (2003).
Clements, J. E. et al. The central nervous system as a reservoir for simian immunodeficiency virus (SIV): steady-state levels of SIV DNA in brain from acute through asymptomatic infection. J. Infect. Dis. 186, 905–913 (2002).
Clements, J. E. et al. The central nervous system is a viral reservoir in simian immunodeficiency virus-infected macaques on combined antiretroviral therapy: a model for human immunodeficiency virus patients on highly active antiretroviral therapy. J. Neurovirol. 11, 180–189 (2005).
Zink, M. C. et al. Simian immunodeficiency virus-infected macaques treated with highly active antiretroviral therapy have reduced central nervous system viral replication and inflammation but persistence of viral DNA. J. Infect. Dis. 202, 161–170 (2010).
Van Rompay, K. K. Evaluation of antiretrovirals in animal models of HIV infection. Antiviral Res. 85, 159–175 (2010).
Deere, J. D. et al. Analysis of multiply spliced transcripts in lymphoid tissue reservoirs of rhesus macaques infected with RT-SHIV during HAART. PLoS ONE 9, e87914 (2014).
North, T. W. et al. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J. Virol. 84, 2913–2922 (2010).
Schmitz, J. E. et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283, 857–860 (1999).
Hansen, S. G. et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science 340, 1237874 (2013).
Hansen, S. G. et al. Immune clearance of highly pathogenic SIV infection. Nature 502, 100–104 (2013).
Mavigner, M. et al. in Special issue: abstracts from the 2014 conference on retroviruses and opportunistic infections. Top. Antivir. Med. Abstract 416 (2014).
Kiem, H. P. et al. in Special issue: abstracts from the 2014 conference on retroviruses and opportunistic infections. Top. Antivir. Med. Abstract 419 (2014).
Spivak, A. M. et al. A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy. Clin. Infect. Dis. 58, 883–890 (2014).
Prins, J. M. et al. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. AIDS 13, 2405–2410 (1999).
Archin, N. M. et al. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res. Hum. Retroviruses 25, 207–212 (2009).
Contreras, X. et al. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J. Biol. Chem. 284, 6782–6789 (2009).
Kauder, S. E., Bosque, A., Lindqvist, A., Planelles, V. & Verdin, E. Epigenetic regulation of HIV-1 latency by cytosine methylation. PLoS Pathog. 5, e1000495 (2009).
Blazkova, J. et al. CpG methylation controls reactivation of HIV from latency. PLoS Pathog. 5, e1000554 (2009).
Bullen, C. K., Laird, G. M., Durand, C. M., Siliciano, J. D. & Siliciano, R. F. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nature Med. 20, 425–429 (2014).
Archin, N. M. et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487, 482–485 (2012).
Shirakawa, K., Chavez, L., Hakre, S., Calvanese, V. & Verdin, E. Reactivation of latent HIV by histone deacetylase inhibitors. Trends Microbiol. 21, 277–285 (2013).
Rasmussen, T. in Special issue: abstracts from the 2014 conference on retroviruses and opportunistic infections. Top. Antivir. Med. 22, e-1 Abstract 438LB (2014).
Elliot, J. et al. in Highlights of the 20th conference on retroviruses and opportunistic infections. Top. Antivir. Med. 21 Abstract 50LB (2013).
Wei, D. G. et al. Histone deacetylase inhibitor romidepsin induces HIV expression in CD4 T cells from patients on suppressive antiretroviral therapy at concentrations achieved by clinical dosing. PLoS Pathog. 10, e1004071 (2014). This study characterizes the ability of the HDAC inhibitor romidepsin to induce HIV-1 expression in vitro in CD4+ T cells isolated from aviraemic patients infected with HIV-1.
Archin, N. M. et al. HIV-1 expression within resting CD4 T-cells following multiple doses of vorinostat. J. Infect. Dis. 210, 728–735 (2014).
Yang, K. et al. in Special issue: abstracts from the 2014 conference on retroviruses and opportunistic infections. Top. Antivir. Med. 22, e-1 Abstract 435 (2014).
Graf, E. H. et al. Gag-positive reservoir cells are susceptible to HIV-specific cytotoxic T lymphocyte mediated clearance. PLoS ONE 8, e71879 (2013).
Ho, Y. C. et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155, 540–551 (2013). This study reports that, although ∼90% of non-induced proviruses in the QVOA are defective, ∼11.7% of these non-induced proviruses have intact genomes, which suggests that the QVOA may underestimate the true size of the latent reservoir in some patients.
Burnett, J. C. et al. Combinatorial latency reactivation for HIV-1 subtypes and variants. J. Virol. 84, 5958–5974 (2010).
Reuse, S. et al. Synergistic activation of HIV-1 expression by deacetylase inhibitors and prostratin: implications for treatment of latent infection. PLoS ONE 4, 0006093 (2009).
Bernhard, W. et al. The Suv39H1 methyltransferase inhibitor chaetocin causes induction of integrated HIV-1 without producing a T cell response. FEBS Lett. 585, 3549–3554 (2011).
Fernandez, G. & Zeichner, S. L. Cell line-dependent variability in HIV activation employing DNMT inhibitors. Virol. J. 7, 266 (2010).
Chou, T. C. Drug combination studies and their synergy quantification using the Chou–Talalay method. Cancer Res. 70, 440–446 (2010).
Shan, L. et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 36, 491–501 (2012). This study found that reversal of HIV-1 latency did not result in uniform cell clearance in an in vitro model system and shows that stimulation of CTLs with HIV-1 peptides improves the recognition and killing of infected cells in vitro.
Khaitan, A. & Unutmaz, D. Revisiting immune exhaustion during HIV infection. Curr. HIV/AIDS Rep. 8, 4–11 (2011).
Moir, S. et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 205, 1797–1805 (2008).
Goulder, P. J. & Watkins, D. I. HIV and SIV CTL escape: implications for vaccine design. Nature Rev. Immunol. 4, 630–640 (2004).
Papuchon, J. et al. Resistance mutations and CTL epitopes in archived HIV-1 DNA of patients on antiviral treatment: toward a new concept of vaccine. PLoS ONE 8, e69029 (2013).
Garcia, F., Leon, A., Gatell, J. M., Plana, M. & Gallart, T. Therapeutic vaccines against HIV infection. Hum. Vaccin. Immunother. 8, 569–581 (2012). This review summarizes the different types of HIV-1 vaccines that have been developed so far, highlighting the main characteristics of each and clinical trials when applicable.
Gay, C. et al. in Special issue: abstracts from the 2014 conference on retroviruses and opportunistic infections. Top. Antivir. Med. 22, e-1 Abstract 344 (2014).
Casazza, J. P. et al. Therapeutic vaccination expands and improves the function of the HIV-specific memory T-cell repertoire. J. Infect. Dis. 207, 1829–1840 (2013).
Chapuis, A. G. et al. HIV-specific CD8+ T cells from HIV+ individuals receiving HAART can be expanded ex vivo to augment systemic and mucosal immunity in vivo. Blood 117, 5391–5402 (2011).
Sung, J. et al. in Sixth International Workshop on HIV Persistence during Therapy Abstract 58 (IHL Press, 2013).
Vivier, E. et al. Innate or adaptive immunity? The example of natural killer cells. Science 331, 44–49 (2011).
Groscurth, P. Cytotoxic effector cells of the immune system. Anat. Embryol. 180, 109–119 (1989).
Hudspeth, K. et al. Engagement of NKp30 on Vδ1 T cells induces the production of CCL3, CCL4, and CCL5 and suppresses HIV-1 replication. Blood 119, 4013–4016 (2012).
Terme, M., Ullrich, E., Delahaye, N. F., Chaput, N. & Zitvogel, L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nature Immunol. 9, 486–494 (2008).
Steel, J. C., Waldmann, T. A. & Morris, J. C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 33, 35–41 (2012).
Wiernik, A. et al. Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16 x 33 bispecific killer cell engager and ADAM17 inhibition. Clin. Cancer Res. 19, 3844–3855 (2013).
Bonsignori, M. et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J. Virol. 86, 11521–11532 (2012).
Porter, D. L., Levine, B. L., Kalos, M., Bagg, A. & June, C. H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 (2011).
Varela-Rohena, A. et al. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nature Med. 14, 1390–1395 (2008). In this study, T cells were engineered to recognize HIV-1 strains that have evaded the immune system.
Heslop, H. E. Genetic engineering of T-cell receptors: TCR takes to titin. Blood 122, 853–854 (2013).
Day, C. L. et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443, 350–354 (2006).
Leng, Q., Bentwich, Z., Magen, E., Kalinkovich, A. & Borkow, G. CTLA-4 upregulation during HIV infection: association with anergy and possible target for therapeutic intervention. AIDS 16, 519–529 (2002).
Palmer, B. E. et al. In vivo blockade of the PD-1 receptor suppresses HIV-1 viral loads and improves CD4+ T cell levels in humanized mice. J. Immunol. 190, 211–219 (2013).
Pincus, S. H. Therapeutic potential of anti-HIV immunotoxins. Antiviral Res. 33, 1–9 (1996).
Davey, R. T. Jr et al. Use of recombinant soluble CD4 Pseudomonas exotoxin, a novel immunotoxin, for treatment of persons infected with human immunodeficiency virus. J. Infect. Dis. 170, 1180–1188 (1994).
Bera, T. K. et al. Specific killing of HIV-infected lymphocytes by a recombinant immunotoxin directed against the HIV-1 envelope glycoprotein. Mol. Med. 4, 384–391 (1998).
Dadachova, E. et al. Targeted killing of virally infected cells by radiolabeled antibodies to viral proteins. PLoS Med. 3, e427 (2006).
Tebas, P. et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N. Engl. J. Med. 370, 901–910 (2014).
Gaj, T., Gersbach, C. A. & Barbas, C. F. 3rd. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 31, 397–405 (2013).
Hatano, H. et al. Prospective antiretroviral treatment of asymptomatic, HIV-1 infected controllers. PLoS Pathog. 9, 10 (2013).
Myers, L. E., McQuay, L. J. & Hollinger, F. B. Dilution assay statistics. J. Clin. Microbiol. 32, 732–739 (1994).
Siliciano, J. D. & Siliciano, R. F. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol. Biol. 304, 3–15 (2005).
Archin, N. M. et al. Special issue: abstracts from the 2014 conference on retroviruses and opportunistic infections. Top. Antivir. Med. 22, e-1 Abstract 406 (2014).
Rouzioux, C. & Richman, D. How to best measure HIV reservoirs? Curr. Opin. HIV AIDS 8, 170–175 (2013).
Pasternak, A. O., Lukashov, V. V. & Berkhout, B. Cell-associated HIV RNA: a dynamic biomarker of viral persistence. Retrovirology 10, 1742–4690 (2013).
Palmer, S. et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J. Clin. Microbiol. 41, 4531–4536 (2003).
Strain, M. C. & Richman, D. D. New assays for monitoring residual HIV burden in effectively treated individuals. Curr. Opin. HIV AIDS 8, 106–110 (2013).
Strain, M. C. et al. Highly precise measurement of HIV DNA by droplet digital PCR. PLoS ONE 8, e55943 (2013). This paper demonstrates the ability of droplet digital PCR to reliably and accurately measure the frequency of total HIV-1 DNA and episomal 2-LTR circles in cells isolated from patients infected with HIV-1.
Kiselinova, M. et al. Comparison of droplet digital PCR and seminested real-time PCR for quantification of cell-associated HIV-1 RNA. PLoS ONE 9, e85999 (2014). This paper reports the feasibility of using droplet digital PCR to measure cell-associated RNA from patients infected with HIV-1.
Sanchez, G., Xu, X., Chermann, J. C. & Hirsch, I. Accumulation of defective viral genomes in peripheral blood mononuclear cells of human immunodeficiency virus type 1-infected individuals. J. Virol. 71, 2233–2240 (1997).
Kieffer, T. L. et al. G→A hypermutation in protease and reverse transcriptase regions of human immunodeficiency virus type 1 residing in resting CD4+ T cells in vivo. J. Virol. 79, 1975–1980 (2005).
Eriksson, S. et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 9, e1003174 (2013).
Anderson, J. A. et al. Clonal sequences recovered from plasma from patients with residual HIV-1 viremia and on intensified antiretroviral therapy are identical to replicating viral RNAs recovered from circulating resting CD4+ T cells. J. Virol. 85, 5220–5223 (2011).
Soriano-Sarabia, N. et al. The quantitation of replication-competent HIV-1 in populations of resting CD4+ T cells. J. Virol. http://dx.doi.org/10.1128/JVI.01900-14 (2014).
Acknowledgements
The authors thank N. Goonetilleke for valuable input. Work in the authors' laboratories was supported by the National Institutes of Health grants AI096113, AI095052 and DA030156 to D.M.M. and AI50410 to the University of North Carolina Center for AIDS research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
D.M.M. is a consultant for or clinical trial support from Merck, GlaxoSmithKline, Viiv Healthcare, Gilead, Bristol-Myers Squibb (BMS), Janssen Pharmaceuticals and Argos Therapeutics; he is also a common stockholder of Gilead. N.M.A., J.M.S., C.G. and N.S.-S. declare no competing interests.
Related links
Glossary
- Activated CD4+ T cells
-
Antigen-specific CD4+ T cells that have undergone stimulation of their T cell receptor–CD3 complexes. Activation of a T cell increases the surface expression of many proteins, including CD69 and CD25, and induces functional responses, such as proliferation and cytokine production.
- HIV-1 reservoirs
-
Infected cell populations that enable the persistence of replication-competent HIV-1 in patients treated with antiretroviral therapy regimens in the order of years. The HIV-1 reservoir comprises both latent HIV-1 infection and other as-yet incompletely defined sources of persistent HIV-1.
- Resting memory CD4+ T cells
-
Antigen-specific CD4+ T cells that have reverted to the G0 state of the cell cycle from a previously activated state but retain the capability to rapidly respond to a second antigenic exposure.
- Latent HIV-1
-
Quiescent, replication-competent provirus that exists within a long-lived population of resting cells and that is capable of initiating new rounds of infection if therapy is interrupted.
- Two-long terminal repeat circles
-
(2-LTR circles). The joining of the two ends of the linear unintegrated HIV-1 DNA (each end having a complete long terminal repeat) into a circularized form of DNA.
- Homeostatic proliferation
-
A physiological process that controls stable and constant cell number without cellular differentiation. Interleukin-7 has a crucial role in maintaining normal T cell levels.
- Central memory CD4+ T cells
-
(TCM cells). Antigen-specific CD4+ T cells that lack immediate effector function but that mediate rapid recall responses and have the capacity to migrate from the blood to the secondary lymphoid organs.
- Transitional memory CD4+ T cells
-
(TTM cells). Antigen-specific T cells that transition to the effector memory state and have lost the surface expression of the homing receptor CC-chemokine receptor 7 but retain the expression of the tumour necrosis factor receptor CD27.
- CD34+ haematopoietic progenitors
-
Human haematopoietic cells that give rise to the myeloid and lymphoid lineages and can be identified by the expression of CD34, CD150 and CD48, but that lack CD244. These cells typically comprise 5% of the total cell population in the bone marrow.
- CD4+ memory stem cells
-
(TSCM cells). Antigen-specific T cells with a broadly naive phenotype but with high surface expression of CD95 (also known as Fas ligand), which is a type II transmembrane protein that is expressed at high levels by all memory cells.
- Chou–Talalay method
-
A method in which a combination index (CI) is used to express the synergy of drugs. A CI < 1 indicates synergy, a CI = 1 suggests an additive effect and a CI > 1 is indicative of antagonism.
Rights and permissions
About this article
Cite this article
Archin, N., Sung, J., Garrido, C. et al. Eradicating HIV-1 infection: seeking to clear a persistent pathogen. Nat Rev Microbiol 12, 750–764 (2014). https://doi.org/10.1038/nrmicro3352
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro3352
This article is cited by
-
Trispecific antibody targeting HIV-1 and T cells activates and eliminates latently-infected cells in HIV/SHIV infections
Nature Communications (2023)
-
Multiplexed shRNA-miRs as a candidate for anti HIV-1 therapy: strategies, challenges, and future potential
Journal of Genetic Engineering and Biotechnology (2022)
-
Engaging innate immunity in HIV-1 cure strategies
Nature Reviews Immunology (2022)
-
Ethics of HIV cure research: an unfinished agenda
BMC Medical Ethics (2021)
-
Ethical considerations for HIV remission clinical research involving participants diagnosed during acute HIV infection
BMC Medical Ethics (2021)