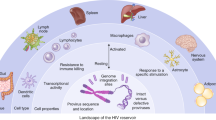
Key Points
-
Interferon-α (IFNα) treatment of cultured primary CD4+ T cells and macrophages potently inhibits HIV-1 replication.
-
IFNα treatment upregulates the expression of the currently identified HIV-1 and SIV restriction factors — APOBEC3G (apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G), TRIM5α (tripartite motif-containing protein 5α), tetherin and SAMHD1 (SAM and HD domain-containing protein 1) — and of the known HIV-1 resistance factors myxovirus resistance 2 (MX2), schlafen 11 (SLFN11) and IFN-induced transmembrane (IFITM) proteins.
-
An intact type I IFN system seems to be crucial for the control of acute lentiviral infection, and IFN treatment suppresses initial SIV infection of macaques.
-
IFNα treatment of patients infected with HIV-1 decreases viral load.
-
In pathogenic, but not in non-pathogenic, non-human primate lentiviral models, there is evidence of persistent IFN signalling.
-
CD4+ T cells of patients chronically infected with HIV-1 show evidence of persistent upregulation of IFN-stimulated genes.
-
Understanding the relationship between HIV-1 infection and the IFN system will yield valuable insights into HIV-1 pathogenesis and will aid the future development of therapeutics.
Abstract
The ability of interferons (IFNs) to inhibit HIV-1 replication in cell culture models has long been recognized, and the therapeutic administration of IFNα to HIV-1-infected patients who are not receiving antiretroviral therapy produces a clear but transient decrease in plasma viral load. Conversely, studies of chronic HIV-1 infection in humans and SIV-infected animal models of AIDS show positive correlations between elevated plasma levels of IFNs, increased expression of IFN-stimulated genes (ISGs), biomarkers of inflammation and disease progression. In this Review, we discuss the evidence that IFNs can control HIV-1 replication in vivo and debate the controversial role of IFNs in promoting the pathological sequelae of chronic HIV-1 infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Faria, N. R. et al. HIV epidemiology. The early spread and epidemic ignition of HIV-1 in human populations. Science 346, 56–61 (2014).
D'Arc, M. et al. Origin of the HIV-1 group O epidemic in western lowland gorillas. Proc. Natl Acad. Sci. USA 112, E1343–E1352 (2015).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Rotger, M. et al. Genome-wide mRNA expression correlates of viral control in CD4+ T-cells from HIV-1-infected individuals. PLoS Pathog. 6, e1000781 (2010). This study characterizes the gene expression profile abnormalities of the CD4+ T cells of patients with HIV-1 infection according to plasma viral load.
Hyrcza, M. D. et al. Distinct transcriptional profiles in ex vivo CD4+ and CD8+ T cells are established early in human immunodeficiency virus type 1 infection and are characterized by a chronic interferon response as well as extensive transcriptional changes in CD8+ T cells. J. Virol. 81, 3477–3486 (2007).
Sedaghat, A. R. et al. Chronic CD4+ T-cell activation and depletion in human immunodeficiency virus type 1 infection: type I interferon-mediated disruption of T-cell dynamics. J. Virol. 82, 1870–1883 (2008).
Chelbi-Alix, M. K. & Wietzerbin, J. Interferon, a growing cytokine family: 50 years of interferon research. Biochimie 89, 713–718 (2007).
Prokunina-Olsson, L. et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nature Genet. 45, 164–171 (2013).
Takaoka, A. & Yanai, H. Interferon signalling network in innate defence. Cell. Microbiol. 8, 907–922 (2006).
McNab, F., Mayer-Barber, K., Sher, A., Wack, A. & O'Garra, A. Type I interferons in infectious disease. Nature Rev. Immunol. 15, 87–103 (2015).
de Veer, M. J. et al. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukoc. Biol. 69, 912–920 (2001).
MacMicking, J. D. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nature Rev. Immunol. 12, 367–382 (2012).
Schoggins, J. W. Interferon-stimulated genes: roles in viral pathogenesis. Curr. Opin. Virol. 6, 40–46 (2014).
Schoggins, J. W. et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472, 481–485 (2011).
Liu, S. Y., Sanchez, D. J., Aliyari, R., Lu, S. & Cheng, G. Systematic identification of type I and type II interferon-induced antiviral factors. Proc. Natl Acad. Sci. USA 109, 4239–4244 (2012).
Schoggins, J. W. et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 505, 691–695 (2014).
Goubau, D., Deddouche, S. & Reis e Sousa, C. Cytosolic sensing of viruses. Immunity 38, 855–869 (2013).
Gao, D. et al. Cyclic GMP–AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 341, 903–906 (2013). This study identifies cGAS as an important sensor of HIV.
Monroe, K. M. et al. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science 343, 428–432 (2014).
Lahaye, X. et al. The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. Immunity 39, 1132–1142 (2013).
Rasaiyaah, J. et al. HIV-1 evades innate immune recognition through specific cofactor recruitment. Nature 503, 402–405 (2013).
Jakobsen, M. R. et al. IFI16 senses DNA forms of the lentiviral replication cycle and controls HIV-1 replication. Proc. Natl Acad. Sci. USA 110, E4571–E4580 (2013).
Matreyek, K. A. & Engelman, A. Viral and cellular requirements for the nuclear entry of retroviral preintegration nucleoprotein complexes. Viruses 5, 2483–2511 (2013).
Unterholzner, L. et al. IFI16 is an innate immune sensor for intracellular DNA. Nature Immunol. 11, 997–1004 (2010).
Doitsh, G. et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505, 509–514 (2014).
Doitsh, G. et al. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell 143, 789–801 (2010). This paper reports that the majority of CD4+ T cells depleted from HIV-1-infected tissues are abortively infected resting cells that die via IFI16-dependent pyroptosis.
Beignon, A. S. et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor–viral RNA interactions. J. Clin. Invest. 115, 3265–3275 (2005).
Lepelley, A. et al. Innate sensing of HIV-infected cells. PLoS Pathog. 7, e1001284 (2011).
Blasius, A. L. & Beutler, B. Intracellular Toll-like receptors. Immunity 32, 305–315 (2010).
Li, G. et al. Plasmacytoid dendritic cells suppress HIV-1 replication but contribute to HIV-1 induced immunopathogenesis in humanized mice. PLoS Pathog. 10, e1004291 (2014).
Bruel, T. et al. Plasmacytoid dendritic cell dynamics tune interferon-alfa production in SIV-infected cynomolgus macaques. PLoS Pathog. 10, e1003915 (2014).
Kader, M. et al. Blocking TLR7- and TLR9-mediated IFN-α production by plasmacytoid dendritic cells does not diminish immune activation in early SIV infection. PLoS Pathog. 9, e1003530 (2013).
Tsang, J. et al. HIV-1 infection of macrophages is dependent on evasion of innate immune cellular activation. AIDS 23, 2255–2263 (2009).
Yan, N., Regalado-Magdos, A. D., Stiggelbout, B., Lee-Kirsch, M. A. & Lieberman, J. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nature Immunol. 11, 1005–1013 (2010).
Manel, N. & Littman, D. R. Hiding in plain sight: how HIV evades innate immune responses. Cell 147, 271–274 (2011).
Pertel, T. et al. TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature 472, 361–365 (2011).
Galao, R. P., Le Tortorec, A., Pickering, S., Kueck, T. & Neil, S. J. Innate sensing of HIV-1 assembly by tetherin induces NFκB-dependent proinflammatory responses. Cell Host Microbe 12, 633–644 (2012).
Galao, R. P., Pickering, S., Curnock, R. & Neil, S. J. Retroviral retention activates a Syk-dependent HemITAM in human tetherin. Cell Host Microbe 16, 291–303 (2014).
Neil, S. & Bieniasz, P. Human immunodeficiency virus, restriction factors, and interferon. J. Interferon Cytokine Res. 29, 569–580 (2009).
Yan, N. & Chen, Z. J. Intrinsic antiviral immunity. Nature Immunol. 13, 214–222 (2012).
Sheehy, A. M., Gaddis, N. C., Choi, J. D. & Malim, M. H. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418, 646–650 (2002).
Stremlau, M. et al. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature 427, 848–853 (2004).
Laguette, N. et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474, 654–657 (2011).
Hrecka, K. et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474, 658–661 (2011).
Neil, S. J., Zang, T. & Bieniasz, P. D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451, 425–430 (2008).
Van Damme, N. et al. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3, 245–252 (2008).
Malim, M. H. & Bieniasz, P. D. HIV restriction factors and mechanisms of evasion. Cold Spring Harb. Perspect. Med. 2, a006940 (2012). This paper provides an overview of HIV restriction factors.
Schaller, T., Goujon, C. & Malim, M. H. AIDS/HIV. HIV interplay with SAMHD1. Science 335, 1313–1314 (2012).
Goldstone, D. C. et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 480, 379–382 (2011).
Lahouassa, H. et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nature Immunol. 13, 223–228 (2011).
Ryoo, J. et al. The ribonuclease activity of SAMHD1 is required for HIV-1 restriction. Nature Med. 20, 936–941 (2014).
Kirchhoff, F. Immune evasion and counteraction of restriction factors by HIV-1 and other primate lentiviruses. Cell Host Microbe 8, 55–67 (2010).
Sharp, P. M. & Hahn, B. H. Origins of HIV and the AIDS pandemic. Cold Spring Harb. Perspect. Med. 1, a006841 (2011).
Yu, X. et al. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif–Cul5–SCF complex. Science 302, 1056–1060 (2003).
Guo, Y. et al. Structural basis for hijacking CBF-β and CUL5 E3 ligase complex by HIV-1 Vif. Nature 505, 229–233 (2014).
Neil, S. J. The antiviral activities of tetherin. Curr. Top. Microbiol. Immunol. 371, 67–104 (2013).
Diamond, T. L. et al. Macrophage tropism of HIV-1 depends on efficient cellular dNTP utilization by reverse transcriptase. J. Biol. Chem. 279, 51545–51553 (2004).
Pillai, S. K. et al. Role of retroviral restriction factors in the interferon-α-mediated suppression of HIV-1 in vivo. Proc. Natl Acad. Sci. USA 109, 3035–3040 (2012).
Armitage, A. E. et al. Conserved footprints of APOBEC3G on hypermutated human immunodeficiency virus type 1 and human endogenous retrovirus HERV-K(HML2) sequences. J. Virol. 82, 8743–8761 (2008).
Wood, N. et al. HIV evolution in early infection: selection pressures, patterns of insertion and deletion, and the impact of APOBEC. PLoS Pathog. 5, e1000414 (2009).
Kim, E. Y. et al. Human APOBEC3 induced mutation of human immunodeficiency virus type-1 contributes to adaptation and evolution in natural infection. PLoS Pathog. 10, e1004281 (2014).
Albin, J. S. & Harris, R. S. Interactions of host APOBEC3 restriction factors with HIV-1 in vivo: implications for therapeutics. Expert Rev. Mol. Med. 12, e4 (2010).
Neil, S. J., Sandrin, V., Sundquist, W. I. & Bieniasz, P. D. An interferon-α-induced tethering mechanism inhibits HIV-1 and Ebola virus particle release but is counteracted by the HIV-1 Vpu protein. Cell Host Microbe 2, 193–203 (2007).
Ho, D. D. et al. Recombinant human interferon alfa-A suppresses HTLV-III replication in vitro. Lancet 326, 602–604 (1985).
Kornbluth, R. S., Oh, P. S., Munis, J. R., Cleveland, P. H. & Richman, D. D. Interferons and bacterial lipopolysaccharide protect macrophages from productive infection by human immunodeficiency virus in vitro. J. Exp. Med. 169, 1137–1151 (1989).
Shirazi, Y. & Pitha, P. M. Alpha interferon inhibits early stages of the human immunodeficiency virus type 1 replication cycle. J. Virol. 66, 1321–1328 (1992).
Meylan, P. R., Guatelli, J. C., Munis, J. R., Richman, D. D. & Kornbluth, R. S. Mechanisms for the inhibition of HIV replication by interferons-α, -β, and -γ in primary human macrophages. Virology 193, 138–148 (1993).
Goujon, C. & Malim, M. H. Characterization of the alpha interferon-induced postentry block to HIV-1 infection in primary human macrophages and T cells. J. Virol. 84, 9254–9266 (2010).
Cheney, K. M. & McKnight, A. Interferon-α mediates restriction of human immunodeficiency virus type-1 replication in primary human macrophages at an early stage of replication. PLoS ONE 5, e13521 (2010).
Goujon, C. et al. Human MX2 is an interferon-induced post-entry inhibitor of HIV-1 infection. Nature 502, 559–562 (2013).
Liu, Z. et al. The interferon-inducible MxB protein inhibits HIV-1 infection. Cell Host Microbe 14, 398–410 (2013).
Kane, M. et al. MX2 is an interferon-induced inhibitor of HIV-1 infection. Nature 502, 563–566 (2013). References 70–72 describe the anti-HIV-1 activities of MX2.
Melen, K. et al. Human MxB protein, an interferon-α-inducible GTPase, contains a nuclear targeting signal and is localized in the heterochromatin region beneath the nuclear envelope. J. Biol. Chem. 271, 23478–23486 (1996).
Goujon, C. et al. Transfer of the amino-terminal nuclear envelope targeting domain of human MX2 converts MX1 into an HIV-1 resistance factor. J. Virol. 88, 9017–9026 (2014).
Fricke, T. et al. MxB binds to the HIV-1 core and prevents the uncoating process of HIV-1. Retrovirology 11, 68 (2014).
Fribourgh, J. L. et al. Structural insight into HIV-1 restriction by MxB. Cell Host Microbe 16, 627–638 (2014).
Compton, A. A. et al. IFITM proteins incorporated into HIV-1 virions impair viral fusion and spread. Cell Host Microbe 16, 736–747 (2014).
Tartour, K. et al. IFITM proteins are incorporated onto HIV-1 virion particles and negatively imprint their infectivity. Retrovirology 11, 103 (2014).
Lu, J. et al. The IFITM proteins inhibit HIV-1 infection. J. Virol. 85, 2126–2137 (2011).
Li, M. et al. Codon-usage-based inhibition of HIV protein synthesis by human schlafen 11. Nature 491, 125–128 (2012).
Alsharifi, M., Mullbacher, A. & Regner, M. Interferon type I responses in primary and secondary infections. Immunol. Cell Biol. 86, 239–245 (2008).
Sandler, N. G. et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature 511, 601–605 (2014). This paper highlights the importance of type I interferons in the control of acute SIV infection.
Stacey, A. R. et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J. Virol. 83, 3719–3733 (2009).
Parrish, N. F. et al. Phenotypic properties of transmitted founder HIV-1. Proc. Natl Acad. Sci. USA 110, 6626–6633 (2013).
Fenton-May, A. E. et al. Relative resistance of HIV-1 founder viruses to control by interferon-α. Retrovirology 10, 146 (2013).
Lane, H. C. et al. Interferon-α in patients with asymptomatic human immunodeficiency virus (HIV) infection: a randomized, placebo-controlled trial. Ann. Intern. Med. 112, 805–811 (1990).
Torriani, F. J. et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N. Engl. J. Med. 351, 438–450 (2004).
Neumann, A. et al. Differential antiviral effect of PEG-interferon-α-2b on HIV and HCV in the treatment of HIV/HCV co-infected patients. AIDS 21, 1855–1865 (2007).
Asmuth, D. M. et al. Safety, tolerability, and mechanisms of antiretroviral activity of pegylated interferon alfa-2a in HIV-1-monoinfected participants: a Phase II clinical trial. J. Infect. Dis. 201, 1686–1696 (2010). This study clearly characterizes the effect of IFNα therapy on HIV-1 plasma viral load in patients infected with HIV-1.
Hubbard, J. J. et al. Host gene expression changes correlating with anti-HIV-1 effects in human subjects after treatment with peginterferon alfa-2a. J. Infect. Dis. 205, 1443–1447 (2012).
Sarasin-Filipowicz, M. et al. Interferon signaling and treatment outcome in chronic hepatitis C. Proc. Natl Acad. Sci. USA 105, 7034–7039 (2008).
Vanderford, T. H. et al. Treatment of SIV-infected sooty mangabeys with a type-I IFN agonist results in decreased virus replication without inducing hyperimmune activation. Blood 119, 5750–5757 (2012).
Azzoni, L. et al. Pegylated interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J. Infect. Dis. 207, 213–222 (2013).
Sun, H. et al. Hepatitis C therapy with interferon-α and ribavirin reduces CD4 T-cell-associated HIV-1 DNA in HIV-1/hepatitis C virus-coinfected patients. J. Infect. Dis. 209, 1315–1320 (2014).
Barouch, D. H. & Deeks, S. G. Immunologic strategies for HIV-1 remission and eradication. Science 345, 169–174 (2014).
Hardy, G. A. et al. Interferon-α is the primary plasma type-I IFN in HIV-1 infection and correlates with immune activation and disease markers. PLoS ONE 8, e56527 (2013).
Herbeuval, J. P. et al. CD4+ T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent, TRAIL/DR5-mediated apoptosis. Blood 106, 3524–3531 (2005).
Douek, D. C., Roederer, M. & Koup, R. A. Emerging concepts in the immunopathogenesis of AIDS. Annu. Rev. Med. 60, 471–484 (2009).
Marshall, H. D., Urban, S. L. & Welsh, R. M. Virus-induced transient immune suppression and the inhibition of T cell proliferation by type I interferon. J. Virol. 85, 5929–5939 (2011).
Marshall, H. D. & Kaech, S. M. Regulating the diverse outcomes of interferon's interference. Trends Immunol. 35, 353–354 (2014).
Fraietta, J. A. et al. Type I interferon upregulates Bak and contributes to T cell loss during human immunodeficiency virus (HIV) infection. PLoS Pathog. 9, e1003658 (2013).
Bosinger, S. E. et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J. Clin. Invest. 119, 3556–3572 (2009).
Jacquelin, B. et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J. Clin. Invest. 119, 3544–3555 (2009).
Harris, L. D. et al. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J. Virol. 84, 7886–7891 (2010). References 102–104 identify the differences in the type I IFN responses of pathogenic and non-pathogenic NHP models following SIV infection.
Jacquelin, B. et al. Innate immune responses and rapid control of inflammation in African green monkeys treated or not with interferon-α during primary SIVagm infection. PLoS Pathog. 10, e1004241 (2014).
Best, S., Le Tissier, P., Towers, G. & Stoye, J. P. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature 382, 826–829 (1996).
Paolini, R., Bernardini, G., Molfetta, R. & Santoni, A. NK cells and interferons. Cytokine Growth Factor Rev. http://dx.doi.org/10.1016/j.cytogfr.2014.11.003 (2014).
Swann, J. B. et al. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J. Immunol. 178, 7540–7549 (2007).
Zanoni, I. et al. IL-15 cis presentation is required for optimal NK cell activation in lipopolysaccharide-mediated inflammatory conditions. Cell Rep. 4, 1235–1249 (2013).
Kolumam, G. A., Thomas, S., Thompson, L. J., Sprent, J. & Murali-Krishna, K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202, 637–650 (2005).
Bahl, K., Huebner, A., Davis, R. J. & Welsh, R. M. Analysis of apoptosis of memory T cells and dendritic cells during the early stages of viral infection or exposure to Toll-like receptor agonists. J. Virol. 84, 4866–4877 (2010).
Srivastava, S., Koch, M. A., Pepper, M. & Campbell, D. J. Type I interferons directly inhibit regulatory T cells to allow optimal antiviral T cell responses during acute LCMV infection. J. Exp. Med. 211, 961–974 (2014).
Le Bon, A. et al. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14, 461–470 (2001).
Hervas-Stubbs, S. et al. Direct effects of type I interferons on cells of the immune system. Clin. Cancer Res. 17, 2619–2627 (2011).
Acknowledgements
The authors are funded by grants and fellowships from the UK Medical Research Council, the European Commission, the Wellcome Trust, the US National Institutes of Health, and the Department of Health via a UK National Institute for Health Research comprehensive Biomedical Research Centre award to Guy's and St. Thomas' National Health Service (NHS) Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Retrovirus
-
A positive-sense single-stranded RNA virus of the family Retroviridae. These viruses replicate via a DNA intermediate that is synthesized by the reverse transcriptase enzyme. Retroviruses integrate their DNA into the host cell chromosome.
- Lentivirus
-
A particular genus of retroviruses that are primarily characterized by infections with long clinical incubation periods, often years to decades. Lentiviruses infect primate and non-primate hosts.
- Set-point plasma viral load
-
The semi-stable plasma level of HIV-1 RNA that is reached after the period of acute HIV-1 infection in most patients, in the absence of antiretroviral therapy.
- Pattern recognition receptors
-
(PRRs). Germ line-encoded receptors that recognize the pathogen-associated molecular patterns which characterize pathogenic microorganisms.
- Pathogen-associated molecular patterns
-
(PAMPs). Biomolecules of diverse nature (ranging from lipopolysaccharides to forms of nucleic acids) that are characteristic of pathogenic microorganisms.
- Apoptosis
-
A mode of programmed cell death that leads to the elimination of the cell without the release of inflammatory mediators.
- Plasmacytoid dendritic cells
-
(pDCs). DCs that are specialized in the detection of microbial pathogens and the production of interferon-α (IFNα). pDCs are thought to be particularly important for HIV-1 sensing.
- Monocyte-derived dendritic cells
-
(MDDCs). DCs that have been derived by inducing their differentiation from primary monocytes in vitro.
- Monocyte-derived macrophages
-
(MDMs). Macrophages that have been derived by inducing their differentiation from primary monocytes in vitro.
- Reverse-transcription complexes
-
(RTCs). Complexes of viral nucleic acid, viral proteins (for example, reverse transcriptase) and cellular proteins that mediate viral DNA synthesis. RTCs are derived from viral capsids following virus entry into the cytoplasm during infection.
- Pyroptosis
-
A mode of programmed cell death that leads to the release of mediators of inflammation and that is often triggered by recognition of pathogenic microorganisms.
- Lymphoid aggregate cultures
-
Cultures composed of small blocks of lymphoid tissue usually derived from the tonsils or the spleen. This experimental system is used in an attempt to replicate the spatial organization and cytokine milieu of in vivo lymphoid tissue.
- Humanized mice
-
Mice that congenitally lack T cells, B cells and natural killer cells, and that are transplanted with human haematopoietic stem cells, leading to the reconstitution of a human-derived immune system.
- Nuclear pore complexes
-
Large protein complexes that form the channels in the nuclear envelope which allow the transport of molecules between the nucleus and the cytoplasm.
- Pegylated
-
Covalently conjugated to polyethylene glycol (PEG). This alters the pharmacokinetic behaviour of a drug, allowing the dosing frequency to be reduced in the case of interferon-α (IFNα).
- Transmitted/founder viruses
-
(T/F viruses). Viruses that are responsible for the establishment of initial HIV-1 infection and from which the viral population seen in later infection is thought to be derived.
- Highly active antiretroviral therapy
-
(HAART). A combination of antiretroviral drugs used to suppress HIV replication.
- Kaposi sarcoma
-
A common tumour associated with advanced HIV-1 infection. The tumour is caused by human herpesvirus 8 (HHV8) and presents as a purplish-brown vascular lesion either on the skin or in internal organs.
- HIV-1 eradication
-
Clearance of replication-competent HIV-1 from the body of an infected person. Achieving this goal is generally thought to require the inhibition of any ongoing HIV-1 replication during conventional antiretroviral therapy and the elimination of infected cells harbouring latent (or transcriptionally inactive) but replication-competent HIV-1. The term reservoir is generally used to denote the pool of latently infected cells.
- Microbial translocation
-
The emergence of microorganisms and microbial products into the portal and systemic circulation from the gut, owing to compromise of the host gastrointestinal immune system.
Rights and permissions
About this article
Cite this article
Doyle, T., Goujon, C. & Malim, M. HIV-1 and interferons: who's interfering with whom?. Nat Rev Microbiol 13, 403–413 (2015). https://doi.org/10.1038/nrmicro3449
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro3449
This article is cited by
-
Innate immune regulation in HIV latency models
Retrovirology (2022)
-
Vpx enhances innate immune responses independently of SAMHD1 during HIV-1 infection
Retrovirology (2021)
-
MX2-mediated innate immunity against HIV-1 is regulated by serine phosphorylation
Nature Microbiology (2021)
-
Unmapped exome reads implicate a role for Anelloviridae in childhood HIV-1 long-term non-progression
npj Genomic Medicine (2021)
-
Increased expression of CDKN1A/p21 in HIV-1 controllers is correlated with upregulation of ZC3H12A/MCPIP1
Retrovirology (2020)