Key Points
-
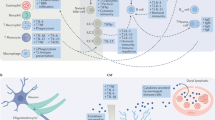
The neurobiology of depression features dichotomous alterations in corticolimbic brain regions. For example, the prefrontal cortex and hippocampus exhibit neuronal atrophy and synaptic dysfunction, whereas the nucleus accumbens and amygdala exhibit neuronal hypertrophy and increased synaptic activity.
-
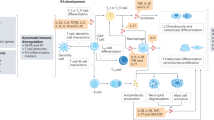
In subsets of depressed individuals, there is dysregulation of peripheral and central immune systems that are implicated in the neurobiology of depression. Rodents exposed to environmental and psychosocial stress recapitulate immune dysfunction observed in clinical populations.
-
Microglia, the brain-resident macrophages, integrate neuroimmune signals and mediate neuroplasticity in physiological and pathological conditions. Neurons provide soluble and contact-dependent signals that modulate the function and activation of microglia.
-
Typical antidepressant agents improve mood by regulating the levels of the monoamines serotonin and noradrenaline, but also partially through attenuation of immune dysregulation. Other antidepressant therapies that limit neuroimmune activation and promote anti-inflammatory pathways may provide alternative treatment options for subsets of depressed individuals.
-
Further studies of the dynamic role of microglia in the neurobiology of depression and synapse function may reveal novel molecular pathways that can be therapeutically targeted.
Abstract
Data from clinical and preclinical studies indicate that immune dysregulation, specifically of inflammatory processes, is associated with symptoms of major depressive disorder (MDD). In particular, increased levels of circulating pro-inflammatory cytokines and concomitant activation of brain-resident microglia can lead to depressive behavioural symptoms. Repeated exposure to psychological stress has a profound impact on peripheral immune responses and perturbs the function of brain microglia, which may contribute to neurobiological changes underlying MDD. Here, we review these findings and discuss ongoing studies examining neuroimmune mechanisms that influence neuronal activity as well as synaptic plasticity. Interventions targeting immune-related cellular and molecular pathways may benefit subsets of MDD patients with immune dysregulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kessler, R. C., Chiu, W. T., Demler, O., Merikangas, K. R. & Walters, E. E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 617–627 (2005).
Kessler, R. C. Epidemiology of women and depression. J. Affect. Disord. 74, 5–13 (2003).
Murray, C. J. et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA 310, 591–608 (2013).
Russo, S. J. & Nestler, E. J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 14, 609–625 (2013).
Krishnan, V. & Nestler, E. J. The molecular neurobiology of depression. Nature 455, 894–902 (2008).
Duman, R. S. & Aghajanian, G. K. Synaptic dysfunction in depression: potential therapeutic targets. Science 338, 68–72 (2012).
Duman, R. S. & Li, N. A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Phil. Trans. R. Soc. B 367, 2475–2484 (2012).
Schmidt, H. D., Shelton, R. C. & Duman, R. S. Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology 36, 2375–2394 (2011).
Foley, D. L. et al. Major depression and associated impairment: same or different genetic and environmental risk factors? Am. J. Psychiatry 160, 2128–2133 (2003).
Sullivan, P. F., Neale, M. C. & Kendler, K. S. Genetic epidemiology of major depression: review and meta-analysis. Am. J. Psychiatry 157, 1552–1562 (2000).
Gilman, S. E. et al. Psychosocial stressors and the prognosis of major depression: a test of Axis IV. Psychol. Med. 43, 303–316 (2013).
McLaughlin, K. A., Conron, K. J., Koenen, K. C. & Gilman, S. E. Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychol. Med. 40, 1647–1658 (2010).
Kendler, K. S. & Halberstadt, L. J. The road not taken: life experiences in monozygotic twin pairs discordant for major depression. Mol. Psychiatry 18, 975–984 (2013).
Duman, R. S. Neuronal damage and protection in the pathophysiology and treatment of psychiatric illness: stress and depression. Dialogues Clin. Neurosci. 11, 239–255 (2009).
Christoffel, D. J., Golden, S. A. & Russo, S. J. Structural and synaptic plasticity in stress-related disorders. Rev. Neurosci. 22, 535–549 (2011).
Hodes, G. E., Kana, V., Menard, C., Merad, M. & Russo, S. J. Neuroimmune mechanisms of depression. Nat. Neurosci. 18, 1386–1393 (2015).
Wohleb, E. S., McKim, D. B., Sheridan, J. F. & Godbout, J. P. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front. Neurosci. 8, 447 (2015).
Maes, M. Evidence for an immune response in major depression: a review and hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry 19, 11–38 (1995).
Howren, M. B., Lamkin, D. M. & Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med. 71, 171–186 (2009).
Dowlati, Y. et al. A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457 (2010).
Capuron, L. & Dantzer, R. Cytokines and depression: the need for a new paradigm. Brain Behav. Immun. 17 (Suppl. 1), 119–124 (2003).
Dantzer, R., O'Connor, J. C., Freund, G. G., Johnson, R. W. & Kelley, K. W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56 (2008).
Miller, A. H., Maletic, V. & Raison, C. L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65, 732–741 (2009).
Iwata, M., Ota, K. T. & Duman, R. S. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav. Immun. 31, 105–114 (2013).
Ridker, P. M., Thuren, T., Zalewski, A. & Libby, P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am. Heart J. 162, 597–605 (2011).
Dunn, J. H., Ellis, L. Z. & Fujita, M. Inflammasomes as molecular mediators of inflammation and cancer: potential role in melanoma. Cancer Lett. 314, 24–33 (2012).
da Silva, J., Goncalves-Pereira, M., Xavier, M. & Mukaetova-Ladinska, E. B. Affective disorders and risk of developing dementia: systematic review. Br. J. Psychiatry 202, 177–186 (2013).
Leonard, B. E. Inflammation, depression and dementia: are they connected? Neurochem. Res. 32, 1749–1756 (2007).
Ownby, R. L., Crocco, E., Acevedo, A., John, V. & Loewenstein, D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch. Gen. Psychiatry 63, 530–538 (2006).
Goodman, W. K. & Charney, D. S. Therapeutic applications and mechanisms of action of monoamine oxidase inhibitor and heterocyclic antidepressant drugs. J. Clin. Psychiatry 46, 6–24 (1985).
Heninger, G. R., Delgado, P. L. & Charney, D. S. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry 29, 2–11 (1996).
Maas, J. W. Biogenic amines and depression. Biochemical and pharmacological separation of two types of depression. Arch. Gen. Psychiatry 32, 1357–1361 (1975).
Kendell, S. F., Krystal, J. H. & Sanacora, G. GABA and glutamate systems as therapeutic targets in depression and mood disorders. Expert Opin. Ther. Targets 9, 153–168 (2005).
Northoff, G. & Sibille, E. Why are cortical GABA neurons relevant to internal focus in depression? A cross-level model linking cellular, biochemical and neural network findings. Mol. Psychiatry 19, 966–977 (2014).
Sanacora, G. & Saricicek, A. GABAergic contributions to the pathophysiology of depression and the mechanism of antidepressant action. CNS Neurol. Disord. Drug Targets 6, 127–140 (2007).
Butler, P. W. & Besser, G. M. Pituitary–adrenal function in severe depressive illness. Lancet 1, 1234–1236 (1968).
Dinan, T. G. Glucocorticoids and the genesis of depressive illness. A psychobiological model. Br. J. Psychiatry 164, 365–371 (1994).
Price, J. L. & Drevets, W. C. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 16, 61–71 (2010).
Ressler, K. J. & Mayberg, H. S. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat. Neurosci. 10, 1116–1124 (2007).
Shin, L. M. & Liberzon, I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35, 169–191 (2010).
Magarinos, A. M., McEwen, B. S., Flugge, G. & Fuchs, E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J. Neurosci. 16, 3534–3540 (1996).
Radley, J. J. et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 125, 1–6 (2004).
Kang, H. J. et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat. Med. 18, 1413–1417 (2012).
Duric, V. et al. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int. J. Neuropsychopharmacol. 16, 69–82 (2012).
Gould, E., Tanapat, P., McEwen, B. S., Flugge, G. & Fuchs, E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc. Natl Acad. Sci. USA 95, 3168–3171 (1998).
Gould, E., McEwen, B. S., Tanapat, P., Galea, L. A. & Fuchs, E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J. Neurosci. 17, 2492–2498 (1997).
Magarinos, A. M. & McEwen, B. S. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 69, 89–98 (1995).
Wellman, C. L. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J. Neurobiol. 49, 245–253 (2001).
Vyas, A., Mitra, R., Shankaranarayana Rao, B. S. & Chattarji, S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 22, 6810–6818 (2002).
Roozendaal, B., McEwen, B. S. & Chattarji, S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 10, 423–433 (2009).
Krishnan, V. et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404 (2007).
Malberg, J. E., Eisch, A. J., Nestler, E. J. & Duman, R. S. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 20, 9104–9110 (2000).
Santarelli, L. et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809 (2003).
Navarria, A. et al. Rapid antidepressant actions of scopolamine: role of medial prefrontal cortex and M1-subtype muscarinic acetylcholine receptors. Neurobiol. Dis. 82, 254–261 (2015).
Voleti, B. et al. Scopolamine rapidly increases Mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol. Psychiatry 74, 742–749 (2013).
Li, N. et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329, 959–964 (2010).
Dantzer, R., O'Connor, J. C., Lawson, M. A. & Kelley, K. W. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology 36, 426–436 (2011).
DellaGioia, N. & Hannestad, J. A critical review of human endotoxin administration as an experimental paradigm of depression. Neurosci. Biobehav. Rev. 34, 130–143 (2010).
Steptoe, A., Hamer, M. & Chida, Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav. Immun. 21, 901–912 (2007).
Felger, J. C. et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry http://dx.doi.org/10.1038/mp.2015.168 (2015). This paper provides initial clinical evidence linking elevated peripheral markers of inflammation with decreases in the functional connectivity of PFC–striatum pathways, which correlate with depressive symptom severity.
Lamers, F. et al. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol. Psychiatry 18, 692–699 (2013). A clinical study showing that subtypes of depression have characteristic immune and hormone biomarkers, potentially reflecting varied pathophysiological mechanisms.
Gold, P. W. The organization of the stress system and its dysregulation in depressive illness. Mol. Psychiatry 20, 32–47 (2015).
Torres-Platas, S. G., Cruceanu, C., Chen, G. G., Turecki, G. & Mechawar, N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav. Immun. 42, 50–59 (2014).
Steiner, J. et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J. Psychiatr. Res. 42, 151–157 (2008).
Setiawan, E. et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72, 268–275 2015).
Martinez, J. M., Garakani, A., Yehuda, R. & Gorman, J. M. Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depress. Anxiety 29, 32–38 (2012).
Carpenter, L. L., Heninger, G. R., Malison, R. T., Tyrka, A. R. & Price, L. H. Cerebrospinal fluid interleukin (IL)-6 in unipolar major depression. J. Affect. Disord. 79, 285–289 (2004).
Hampel, H., Kotter, H. U. & Moller, H. J. Blood–cerebrospinal fluid barrier dysfunction for high molecular weight proteins in Alzheimer disease and major depression: indication for disease subsets. Alzheimer Dis. Assoc. Disord. 11, 78–87 (1997).
Haroon, E. et al. Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol. Psychiatry http://dx.doi.org/10.1038/mp.2015.206 (2016).
Shelton, R. C. et al. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol. Psychiatry 16, 751–762 (2011).
Kim, S., Hwang, Y., Webster, M. J. & Lee, D. Differential activation of immune/inflammatory response-related co-expression modules in the hippocampus across the major psychiatric disorders. Mol. Psychiatry 21, 376–385 (2016).
Beumer, W. et al. The immune theory of psychiatric diseases: a key role for activated microglia and circulating monocytes. J. Leukoc. Biol. 92, 959–975 (2012).
Sternberg, E. M. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 6, 318–328 (2006).
Irwin, M. R. & Cole, S. W. Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol. 11, 625–632 (2011).
Erickson, M. A., Dohi, K. & Banks, W. A. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood–brain barrier. Neuroimmunomodulation 19, 121–130 (2012).
Quan, N. Immune-to-brain signaling: how important are the blood–brain barrier-independent pathways? Mol. Neurobiol. 37, 142–152 (2008).
Schwartz, M., Kipnis, J., Rivest, S. & Prat, A. How do immune cells support and shape the brain in health, disease, and aging? J. Neurosci. 33, 17587–17596 (2013).
Prinz, M., Priller, J., Sisodia, S. S. & Ransohoff, R. M. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 14, 1227–1235 (2011).
Schiltz, J. C. & Sawchenko, P. E. Signaling the brain in systemic inflammation: the role of perivascular cells. Front. Biosci. 8, s1321–s1329 (2003).
Serrats, J. et al. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron 65, 94–106 (2010).
Schwartz, M., London, A. & Shechter, R. Boosting T-cell immunity as a therapeutic approach for neurodegenerative conditions: the role of innate immunity. Neuroscience 158, 1133–1142 (2009).
Kipnis, J., Gadani, S. & Derecki, N. C. Pro-cognitive properties of T cells. Nat. Rev. Immunol. 12, 663–669 (2012).
Dhabhar, F. S., Malarkey, W. B., Neri, E. & McEwen, B. S. Stress-induced redistribution of immune cells — from barracks to boulevards to battlefields: a tale of three hormones — Curt Richter Award winner. Psychoneuroendocrinology 37, 1345–1368 (2012).
Dhabhar, F. S. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation 16, 300–317 (2009).
Trottier, M. D., Newsted, M. M., King, L. E. & Fraker, P. J. Natural glucocorticoids induce expansion of all developmental stages of murine bone marrow granulocytes without inhibiting function. Proc. Natl Acad. Sci. USA 105, 2028–2033 (2008).
Engler, H. et al. Interleukin-1 receptor type 1-deficient mice fail to develop social stress-associated glucocorticoid resistance in the spleen. Psychoneuroendocrinology 33, 108–117 (2008).
Engler, H., Engler, A., Bailey, M. T. & Sheridan, J. F. Tissue-specific alterations in the glucocorticoid sensitivity of immune cells following repeated social defeat in mice. J. Neuroimmunol. 163, 110–119 (2005).
Stark, J. L., Avitsur, R., Hunzeker, J., Padgett, D. A. & Sheridan, J. F. Interleukin-6 and the development of social disruption-induced glucocorticoid resistance. J. Neuroimmunol. 124, 9–15 (2002).
Stark, J. L. et al. Social stress induces glucocorticoid resistance in macrophages. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R1799–R1805 (2001).
Felten, D. L., Felten, S. Y., Carlson, S. L., Olschowka, J. A. & Livnat, S. Noradrenergic and peptidergic innervation of lymphoid tissue. J. Immunol. 135, 755s–765s (1985).
Nance, D. M. & Sanders, V. M. Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav. Immun. 21, 736–745 (2007).
Hanke, M. L., Powell, N. D., Stiner, L. M., Bailey, M. T. & Sheridan, J. F. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav. Immun. 26, 1150–1159 (2012).
Grisanti, L. A. et al. Pro-inflammatory responses in human monocytes are β1-adrenergic receptor subtype dependent. Mol. Immunol. 47, 1244–1254 (2010).
Spiegel, A. et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat. Immunol. 8, 1123–1131 (2007).
Katayama, Y. et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407–421 (2006).
Powell, N. D. et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc. Natl Acad. Sci. USA 110, 16574–16579 (2013).
Heidt, T. et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 20, 754–758 (2014). A preclinical study showing that psychological stress can induce the proliferation and mobilization of haematopoietic immune cells through SNS signalling, resulting in increased numbers of circulating monocytes and granulocytes.
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
Johnson, J. D. et al. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 135, 1295–1307 (2005).
Miller, G. E. et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-κB signaling. Biol. Psychiatry 64, 266–272 (2008).
Pace, T. W. et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry 163, 1630–1633 (2006).
Wohleb, E. S. et al. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J. Neurosci. 34, 2583–2591 (2014).
Wohleb, E. S., Powell, N. D., Godbout, J. P. & Sheridan, J. F. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J. Neurosci. 33, 13820–13833 (2013).
Wohleb, E. S. et al. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology 37, 1491–1505 (2012).
Wohleb, E. S. et al. β-adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J. Neurosci. 31, 6277–6288 (2011).
Hodes, G. E. et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl Acad. Sci. USA 111, 16136–16141 (2014).
McKim, D. B. et al. Sympathetic release of splenic monocytes promotes recurring anxiety following repeated social defeat. Biol. Psychiatry 79, 803–813 (2016).
Wohleb, E. S. et al. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol. Psychiatry 75, 970–981 (2014).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010). Using transgenic mice, this study provides direct evidence that microglia populate the brain early during neurodevelopment and do not undergo renewal from blood-derived myeloid cells.
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758 (2005).
Kettenmann, H., Hanisch, U. K., Noda, M. & Verkhratsky, A. Physiology of microglia. Physiol. Rev. 91, 461–553 (2011).
Ransohoff, R. M. & Perry, V. H. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 27, 119–145 (2009).
Kierdorf, K. & Prinz, M. Factors regulating microglia activation. Front. Cell. Neurosci. 7, 44 (2013).
Butovsky, O. et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 17, 131–143 (2014). This preclinical work reveals that gene expression and function in microglia are driven by brain-specific signals, such as TGFβ, that can contribute to deleterious microglial function.
Elmore, M. R. et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397 (2014). This study uncovers a requirement for CSF1 signalling in maintaining microglial function under physiological conditions, and shows that repopulation of microglia occurs via amplification of intrinsic progenitor cells.
Wang, Y. et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 13, 753–760 (2012).
Biber, K., Neumann, H., Inoue, K. & Boddeke, H. W. Neuronal 'On' and 'Off' signals control microglia. Trends Neurosci. 30, 596–602 (2007).
Jurgens, H. A. & Johnson, R. W. Dysregulated neuronal-microglial cross-talk during aging, stress and inflammation. Exp. Neurol. 233, 40–48 (2010).
Paolicelli, R. C. et al. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458 (2011). Seminal findings showing that microglia contribute to synaptic refinement of hippocampal neurons during development and that reduced microglial function leads to persistence of immature synapses and altered neurodevelopment.
Zhan, Y. et al. Deficient neuron–microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 17, 400–406 (2014).
Rogers, J. T. et al. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J. Neurosci. 31, 16241–16250 (2011).
Tremblay, M. E. et al. The role of microglia in the healthy brain. J. Neurosci. 31, 16064–16069 (2011).
Gyoneva, S. & Traynelis, S. F. Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J. Biol. Chem. 288, 15291–15302 (2013).
Fontainhas, A. M. et al. Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS ONE 6, e15973 (2011).
Pocock, J. M. & Kettenmann, H. Neurotransmitter receptors on microglia. Trends Neurosci. 30, 527–535 (2007).
Tremblay, M. E., Lowery, R. L. & Majewska, A. K. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 8, e1000527 (2010).
Wake, H., Moorhouse, A. J., Jinno, S., Kohsaka, S. & Nabekura, J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 29, 3974–3980 (2009).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Davies, L. C., Jenkins, S. J., Allen, J. E. & Taylor, P. R. Tissue-resident macrophages. Nat. Immunol. 14, 986–995 (2013).
Frank, M. G., Thompson, B. M., Watkins, L. R. & Maier, S. F. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav. Immun. 26, 337–345 (2012).
Frank, M. G., Miguel, Z. D., Watkins, L. R. & Maier, S. F. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav. Immun. 24, 19–30 (2010).
Frank, M. G., Baratta, M. V., Sprunger, D. B., Watkins, L. R. & Maier, S. F. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav. Immun. 21, 47–59 (2007).
Chang, Y. et al. Inhibitory effects of ketamine on lipopolysaccharide-induced microglial activation. Mediators Inflamm. 2009, 705379 (2009).
Nair, A. & Bonneau, R. H. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J. Neuroimmunol. 171, 72–85 (2006).
Glezer, I. & Rivest, S. Glucocorticoids: protectors of the brain during innate immune responses. Neuroscientist 10, 538–552 (2004).
Sorrells, S. F. & Sapolsky, R. M. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav. Immun. 21, 259–272 (2007).
Weber, M. D., Frank, M. G., Sobesky, J. L., Watkins, L. R. & Maier, S. F. Blocking toll-like receptor 2 and 4 signaling during a stressor prevents stress-induced priming of neuroinflammatory responses to a subsequent immune challenge. Brain Behav. Immun. 32, 112–121 (2013).
Pereira, D. B. et al. Life stress, negative mood states, and antibodies to heat shock protein 70 in endometrial cancer. Brain Behav. Immun. 24, 210–214 (2010).
Sriram, K., Rodriguez-Fernandez, M. & Doyle, F. J. III . A detailed modular analysis of heat-shock protein dynamics under acute and chronic stress and its implication in anxiety disorders. PLoS ONE 7, e42958 (2012).
Zlatkovic, J., Bernardi, R. E. & Filipovic, D. Protective effect of Hsp70i against chronic social isolation stress in the rat hippocampus. J. Neural Transm. 121, 3–14 (2014).
Weber, M. D., Frank, M. G., Tracey, K. J., Watkins, L. R. & Maier, S. F. Stress induces the danger-associated molecular pattern HMGB-1 in the hippocampus of male Sprague Dawley rats: a priming stimulus of microglia and the NLRP3 inflammasome. J. Neurosci. 35, 316–324 (2015). Primary findings showing that stress-induced HMGB1 release in the hippocampus causes priming of microglia through increased NLRP3 activation, leading to amplified pro-inflammatory cytokine responses to stimulation by endotoxin.
Wu, T. Y. et al. High-mobility group box-1 was released actively and involved in LPS induced depressive-like behavior. J. Psychiatr. Res. 64, 99–106 (2015).
Emanuele, E. et al. Serum levels of soluble receptor for advanced glycation endproducts (sRAGE) in patients with different psychiatric disorders. Neurosci. Lett. 487, 99–102 (2011).
Aguirre, A., Maturana, C. J., Harcha, P. A. & Saez, J. C. Possible involvement of TLRs and hemichannels in stress-induced CNS dysfunction via mastocytes, and glia activation. Mediators Inflamm. 2013, 893521 (2013).
Verkhratsky, A., Krishtal, O. A. & Burnstock, G. Purinoceptors on neuroglia. Mol. Neurobiol. 39, 190–208 (2009).
Boucsein, C. et al. Purinergic receptors on microglial cells: functional expression in acute brain slices and modulation of microglial activation in vitro. Eur. J. Neurosci. 17, 2267–2276 (2003).
Ogata, T. et al. Adenosine triphosphate inhibits cytokine release from lipopolysaccharide-activated microglia via P2y receptors. Brain Res. 981, 174–183 (2003).
Seo, D. R. et al. Cross talk between P2 purinergic receptors modulates extracellular ATP-mediated interleukin-10 production in rat microglial cells. Exp. Mol. Med. 40, 19–26 (2008).
Harry, G. J. Microglia during development and aging. Pharmacol. Ther. 139, 313–326 (2013).
Basso, A. M. et al. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: relevance for neuropsychiatric disorders. Behav. Brain Res. 198, 83–90 (2009).
Halmai, Z. et al. Associations between depression severity and purinergic receptor P2RX7 gene polymorphisms. J. Affect. Disord. 150, 104–109 (2013).
Lucae, S. et al. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum. Mol. Genet. 15, 2438–2445 (2006).
McQuillin, A. et al. Case–control studies show that a non-conservative amino-acid change from a glutamine to arginine in the P2RX7 purinergic receptor protein is associated with both bipolar- and unipolar-affective disorders. Mol. Psychiatry 14, 614–620 (2009).
Stokes, L. et al. Two haplotypes of the P2X7 receptor containing the Ala-348 to Thr polymorphism exhibit a gain-of-function effect and enhanced interleukin-1β secretion. FASEB J. 24, 2916–2927 (2010).
Li, Y., Du, X. F., Liu, C. S., Wen, Z. L. & Du, J. L. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev. Cell 23, 1189–1202 (2012).
Perrotti, L. I. et al. Induction of ΔFosB in reward-related brain structures after chronic stress. J. Neurosci. 24, 10594–10602 (2004).
Walker, F. R., Nilsson, M. & Jones, K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr. Drug Targets 14, 1262–1276 (2013).
Yirmiya, R., Rimmerman, N. & Reshef, R. Depression as a microglial disease. Trends Neurosci. 38, 637–658 (2015).
Grabert, K. et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 19, 504–516 (2016).
Hinwood, M., Morandini, J., Day, T. A. & Walker, F. R. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb. Cortex 22, 1442–1454 (2012).
Wood, S. K. et al. Inflammatory factors mediate vulnerability to a social stress-induced depressive-like phenotype in passive coping rats. Biol. Psychiatry 78, 38–48 (2015).
Kreisel, T. et al. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol. Psychiatry 19, 699–709 (2014). This study provides compelling findings showing that short-term stress causes microglial activation and that chronic stress exposure promotes dystrophic microglial responses in the hippocampus, both contributing to the development of depressive-like behaviours.
Delpech, J. C. et al. Microglia in neuronal plasticity: influence of stress. Neuropharmacology 96, 19–28 (2015).
Boersma, M. C. et al. A requirement for nuclear factor-κB in developmental and plasticity-associated synaptogenesis. J. Neurosci. 31, 5414–5425 (2011).
Christoffel, D. J. et al. IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J. Neurosci. 31, 314–321 (2011).
Koo, J. W., Russo, S. J., Ferguson, D., Nestler, E. J. & Duman, R. S. Nuclear factor-κB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl Acad. Sci. USA 107, 2669–2674 (2010).
Tanaka, K. et al. Prostaglandin E2-mediated attenuation of mesocortical dopaminergic pathway is critical for susceptibility to repeated social defeat stress in mice. J. Neurosci. 32, 4319–4329 (2012).
Goshen, I. et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatry 13, 717–728 (2008).
Goshen, I. & Yirmiya, R. Interleukin-1 (IL-1): a central regulator of stress responses. Front. Neuroendocrinol. 30, 30–45 (2009).
Koo, J. W. & Duman, R. S. IL-1β is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl Acad. Sci. USA 105, 751–756 (2008).
McKim, D. B. et al. Neuroinflammatory dynamics underlie memory impairments after repeated social defeat. J. Neurosci. 36, 2590–2604 (2016).
Liu, M. et al. Microglia activation regulates GluR1 phosphorylation in chronic unpredictable stress-induced cognitive dysfunction. Stress 18, 96–106 (2015).
Warner-Schmidt, J. L., Vanover, K. E., Chen, E. Y., Marshall, J. J. & Greengard, P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc. Natl Acad. Sci. USA 108, 9262–9267 (2011).
Yirmiya, R. & Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 25, 181–213 (2011).
Yu, H. et al. Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J. Neurosci. 32, 4092–4101 (2012).
Berton, O. et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311, 864–868 (2006).
Parkhurst, C. N. et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609 (2013).
Banasr, M. et al. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol. Psychiatry 15, 501–511 (2010).
Guloksuz, S., Rutten, B. P., Arts, B., van Os, J. & Kenis, G. The immune system and electroconvulsive therapy for depression. J. ECT 30, 132–137 (2014).
Perez-Caballero, L. et al. Early responses to deep brain stimulation in depression are modulated by anti-inflammatory drugs. Mol. Psychiatry 19, 607–614 (2014).
Beattie, E. C. et al. Control of synaptic strength by glial TNFα. Science 295, 2282–2285 (2002). A fundamental study elucidating the mechanisms by which glial-derived TNF modulates baseline levels of synaptic plasticity by trafficking of AMPA receptors.
Stellwagen, D. & Malenka, R. C. Synaptic scaling mediated by glial TNF-α. Nature 440, 1054–1059 (2006).
Stellwagen, D., Beattie, E. C., Seo, J. Y. & Malenka, R. C. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-α. J. Neurosci. 25, 3219–3228 (2005).
Qiu, Z., Sweeney, D. D., Netzeband, J. G. & Gruol, D. L. Chronic interleukin-6 alters NMDA receptor-mediated membrane responses and enhances neurotoxicity in developing CNS neurons. J. Neurosci. 18, 10445–10456 (1998).
Gruol, D. L. & Nelson, T. E. Purkinje neuron physiology is altered by the inflammatory factor interleukin-6. Cerebellum 4, 198–205 (2005).
Qian, J. et al. Interleukin-1R3 mediates interleukin-1-induced potassium current increase through fast activation of Akt kinase. Proc. Natl Acad. Sci. USA 109, 12189–12194 (2012).
Milior, G. et al. Fractalkine receptor deficiency impairs microglial and neuronal responsiveness to chronic stress. Brain Behav. Immun. http://dx.doi.org/10.1016/j.bbi.2015.07.024 (2015). An initial report showing that hippocampal microglial processes have increased dendritic and synaptic elements following chronic stress, and that CX 3 CR1 mediates these synaptic as well as behavioural (anhedonia) deficits.
Walsh, J. G., Muruve, D. A. & Power, C. Inflammasomes in the CNS. Nat. Rev. Neurosci. 15, 84–97 (2014).
Guo, H., Callaway, J. B. & Ting, J. P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 21, 677–687 (2015).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Alcocer-Gomez, E. et al. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav. Immun. 36, 111–117 (2014). Clinical work reporting that depressed individuals have elevated levels of NLRP3 inflammasome proteins in peripheral mononuclear immune cells, suggesting that this is a common molecular pathway leading to enhanced inflammation.
Zhang, Y. et al. NLRP3 inflammasome mediates chronic mild stress-induced depression in mice via neuroinflammation. Int. J. Neuropsychopharmacol. 18, pyv006 (2015).
Alcocer-Gomez, E. et al. Stress-induced depressive behaviors require a functional NLRP3 inflammasome. Mol. Neurobiol. http://dx.doi.org/10.1007/s12035-015-9408-7 (2015).
Iwata, M. et al. Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor. Biol. Psychiatry http://dx.doi.org/10.1016/j.biopsych.2015.11.026 (2015).
Krishnan, V. & Nestler, E. J. Linking molecules to mood: new insight into the biology of depression. Am. J. Psychiatry 167, 1305–1320 (2010).
Walker, F. R. A critical review of the mechanism of action for the selective serotonin reuptake inhibitors: do these drugs possess anti-inflammatory properties and how relevant is this in the treatment of depression? Neuropharmacology 67, 304–317 (2013).
Baune, B. T. & Eyre, H. Anti-inflammatory effects of antidepressant and atypical antipsychotic medication for the treatment of major depression and comorbid arthritis: a case report. J. Med. Case Rep. 4, 6 (2010).
O'Brien, S. M., Scott, L. V. & Dinan, T. G. Antidepressant therapy and C-reactive protein levels. Br. J. Psychiatry 188, 449–452 (2006).
Hannestad, J., DellaGioia, N. & Bloch, M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology 36, 2452–2459 (2011).
Hannestad, J., DellaGioia, N., Ortiz, N., Pittman, B. & Bhagwagar, Z. Citalopram reduces endotoxin-induced fatigue. Brain Behav. Immun. 25, 256–259 (2011).
Tynan, R. J. et al. A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain Behav. Immun. 26, 469–479 (2012).
Ramirez, K., Shea, D. T., McKim, D. B., Reader, B. F. & Sheridan, J. F. Imipramine attenuates neuroinflammatory signaling and reverses stress-induced social avoidance. Brain Behav. Immun. 46, 212–220 (2015).
Maes, M., Song, C. & Yirmiya, R. Targeting IL-1 in depression. Expert Opin. Ther. Targets 16, 1097–1112 (2012).
Raison, C. L. et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70, 31–41 (2013).
Krishnan, R. et al. Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. Br. J. Dermatol. 157, 1275–1277 (2007).
Tyring, S. et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet 367, 29–35 (2006).
Akhondzadeh, S. et al. Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress. Anxiety 26, 607–611 (2009).
Muller, N. et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol. Psychiatry 11, 680–684 (2006).
Na, K. S., Lee, K. J., Lee, J. S., Cho, Y. S. & Jung, H. Y. Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: a meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 48, 79–85 (2014).
Eyre, H. A., Air, T., Proctor, S., Rositano, S. & Baune, B. T. A critical review of the efficacy of non-steroidal anti-inflammatory drugs in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 57, 11–16 (2015).
Fleshner, M. Stress-evoked sterile inflammation, danger associated molecular patterns (DAMPs), microbial associated molecular patterns (MAMPs) and the inflammasome. Brain Behav. Immun. 27, 1–7 (2013).
Yamanashi, T. et al. NLRP3 inflammasome is activated by psychological stress: a potential role of NLRP3 inhibitor β-hydroxybutyrate's antidepressant effect. Program No. 775.05/G34. 2015 Neuroscience Meeting Planner (Society for Neuroscience, Washington, DC, 2015).
Ota, K. T. & Duman, R. S. Environmental and pharmacological modulations of cellular plasticity: role in the pathophysiology and treatment of depression. Neurobiol. Dis. 57, 28–37 (2012).
Eyre, H. A., Papps, E. & Baune, B. T. Treating depression and depression-like behavior with physical activity: an immune perspective. Front. Psychiatry 4, 3 (2013).
Vukovic, J., Colditz, M. J., Blackmore, D. G., Ruitenberg, M. J. & Bartlett, P. F. Microglia modulate hippocampal neural precursor activity in response to exercise and aging. J. Neurosci. 32, 6435–6443 (2012).
Schloesser, R. J., Lehmann, M., Martinowich, K., Manji, H. K. & Herkenham, M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol. Psychiatry 15, 1152–1163 (2010).
Lehmann, M. L., Brachman, R. A., Martinowich, K., Schloesser, R. J. & Herkenham, M. Glucocorticoids orchestrate divergent effects on mood through adult neurogenesis. J. Neurosci. 33, 2961–2972 (2013).
Goshen, I. et al. Environmental enrichment restores memory functioning in mice with impaired IL-1 signaling via reinstatement of long-term potentiation and spine size enlargement. J. Neurosci. 29, 3395–3403 (2009).
DeVries, A. C., Craft, T. K., Glasper, E. R., Neigh, G. N. & Alexander, J. K. 2006 Curt P. Richter award winner: social influences on stress responses and health. Psychoneuroendocrinology 32, 587–603 (2007).
Bailey, M. T. Influence of stressor-induced nervous system activation on the intestinal microbiota and the importance for immunomodulation. Adv. Exp. Med. Biol. 817, 255–276 (2014).
Galley, J. D. & Bailey, M. T. Impact of stressor exposure on the interplay between commensal microbiota and host inflammation. Gut Microbes 5, 390–396 (2014).
Miller, A. H. & Raison, C. L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 16, 22–34 (2015).
Dantzer, R. & Kelley, K. W. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 21, 153–160 (2007).
Acknowledgements
This work was supported by endowment funds from Yale University, New Haven, Connecticut, USA, and by the State of Connecticut, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
R.S.D. has received consulting fees, speaking fees and/or grant support from Naurex, Taisho Pharmaceutical, Johnson & Johnson, Eli Lilly and Company, Lundbeck, Sunovion Pharmaceuticals, and Forest Laboratories. E.S.W., T.F. and M.I. declare no competing interests.
Glossary
- Endotoxin
-
A component of the bacterial cell wall that binds to pattern recognition receptors on host immune cells and elicits inflammatory responses without persistent infection.
- Sickness behaviour
-
Reductions in locomotor activity, food intake and social interaction that are induced by inflammatory factors to facilitate pathogen clearance and recovery, and to prevent spread of infection.
- Anhedonia
-
A core symptom of depression that manifests as an inability to experience pleasure during usually enjoyable activities.
- Melancholic depression
-
A subtype of major depressive disorder that is characterized by anhedonia and diminished affect, leading to impaired mood in response to positive events.
- Atypical depression
-
A subtype of major depressive disorder that is characterized by general fatigue, increased sleep and weight gain, as well as intense changes in mood based on extraneous circumstances and factors.
- Neurovegetative
-
A cluster of depression symptoms, including but not limited to significant changes in weight and eating patterns, sleep patterns and sensitivity to interpersonal issues.
- Social defeat
-
A standardized rodent model of psychosocial stress induced by losing a confrontation with a conspecific.
- Minocycline
-
A brain-penetrating tetracycline antibiotic that exerts anti-inflammatory and neuroprotective effects by putatively directly inhibiting microglia.
- Tricyclic antidepressant
-
An early chemical class of antidepressant drug that acts primarily by inhibiting serotonin and noradrenaline reuptake; however, therapeutic effects lag by several weeks, suggesting a role for adaptive changes.
- Monoamine oxidase inhibitors
-
A class of antidepressant drugs that prevent enzymatic breakdown of monoamines, typically used when other drugs are ineffective.
- β-hydroxybutyrate
-
A ketone metabolite recently reported to selectively block activation of the NLRP3 (NOD-, LRR- and pyrin domain-containing 3) inflammasome, an effect that could underlie the anti-inflammatory effects of ketogenic diets.
Rights and permissions
About this article
Cite this article
Wohleb, E., Franklin, T., Iwata, M. et al. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci 17, 497–511 (2016). https://doi.org/10.1038/nrn.2016.69
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn.2016.69
This article is cited by
-
Effects of lockdowns on neurobiological and psychometric parameters in unipolar depression during the COVID-19 pandemic
Translational Psychiatry (2024)
-
Neurobehavioral protective effects of Japanese sake yeast supplement against chronic stress-induced anxiety and depression-like symptoms in mice: Possible role of central adenosine receptors
Psychopharmacology (2024)
-
Serum indoleamine 2, 3-dioxygenase and tryptophan-2, 3-dioxygenase: potential biomarkers for the diagnosis of major depressive disorder
Psychopharmacology (2024)
-
Identification of novel biomarkers linking depressive disorder and Alzheimer’s disease based on an integrative bioinformatics analysis
BMC Genomic Data (2023)
-
The connexin hemichannel inhibitor D4 produces rapid antidepressant-like effects in mice
Journal of Neuroinflammation (2023)