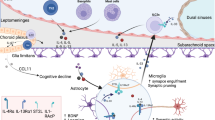
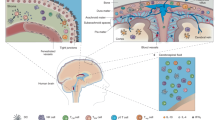
Abstract
The evolution of adaptive immunity provides enhanced defence against specific pathogens, as well as homeostatic immune surveillance of all tissues. Despite being 'immune privileged', the CNS uses the assistance of the immune system in physiological and pathological states. In this Opinion article, we discuss the influence of adaptive immunity on recovery after CNS injury and on cognitive and social brain function. We further extend a hypothesis that the pro-social effects of interferon-regulated genes were initially exploited by pathogens to increase host–host transmission, and that these genes were later recycled by the host to form part of an immune defence programme. In this way, the evolution of adaptive immunity may reflect a host–pathogen 'arms race'.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dantzer, R. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. North Am. 29, 247–264 (2009).
Kipnis, J. Multifaceted interactions between adaptive immunity and the central nervous system. Science 353, 766–771 (2016).
Dendrou, C. A., Fugger, L. & Friese, M. A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 15, 545–558 (2015).
Jander, S., Kraemer, M., Schroeter, M., Witte, O. W. & Stoll, G. Lymphocytic infiltration and expression of intercellular-adhesion molecule-1 in photochemically induced ischemia of the rat cortex. J. Cereb. Blood Flow Metab. 15, 42–51 (1995).
Yilmaz, G., Arumugam, T. V., Stokes, K. Y. & Granger, D. N. Role of T lymphocytes and interferon-γ in ischemic stroke. Circulation 113, 2105–2112 (2006).
Hurn, P. D. et al. T− and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J. Cereb. Blood Flow Metab. 27, 1798–1805 (2007).
Subramanian, S. et al. Recombinant T cell receptor ligand treats experimental stroke. Stroke 40, 2539–2545 (2009).
Luo, Y. et al. Interleukin-33 ameliorates ischemic brain injury in experimental stroke through promoting Th2 response and suppressing Th17 response. Brain Res. 1597, 86–94 (2015).
Zierath, D. et al. The immunologic profile of adoptively transferred lymphocytes influences stroke outcome of recipients. J. Neuroimmunol. 263, 28–34 (2013).
Korhonen, P. et al. Immunomodulation by interleukin-33 is protective in stroke through modulation of inflammation. Brain Behav. Immun. 49, 322–336 (2015).
Serpe, C. J., Kohm, A. P., Huppenbauer, C. B., Sanders, V. M. & Jones, K. J. Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodeficient (scid) mice. J. Neurosci. 19, RC7 (1999).
Kipnis, J. et al. Therapeutic vaccination for closed head injury. J. Neurotrauma 20, 559–569 (2003).
Moalem, G. et al. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat. Med. 5, 49–55 (1999).
Walsh, J. T. et al. MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J. Clin. Invest. 125, 699–714 (2015).
Kipnis, J. et al. Low-dose γ-irradiation promotes survival of injured neurons in the central nervous system via homeostasis-driven proliferation of T cells. Eur. J. Neurosci. 19, 1191–1198 (2004).
Hauben, E. et al. Vaccination with dendritic cells pulsed with peptides of myelin basic protein promotes functional recovery from spinal cord injury. J. Neurosci. 23, 8808–8819 (2003).
Raivich, G. et al. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J. Neurosci. 18, 5804–5816 (1998).
Kipnis, J. et al. Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc. Natl Acad. Sci. USA 26, 15620–15625 (2002).
Kipnis, J. et al. Neuronal survival after CNS insult is determined by a genetically encoded autoimmune response. J. Neurosci. 21, 4564–4571 (2001).
Serpe, C. J., Sanders, V. M. & Jones, K. J. Kinetics of facial motoneuron loss following facial nerve transection in severe combined immunodeficient mice. J. Neurosci. Res. 62, 273–278 (2000).
Barouch, R. & Schwartz, M. Autoreactive T cells induce neurotrophin production by immune and neural cells in injured rat optic nerve: implications for protective autoimmunity. FASEB J. 16, 1304–1306 (2002).
Kipnis, J. et al. T cell immunity to copolymer 1 confers neuroprotection on the damaged optic nerve: possible therapy for optic neuropathies. Proc. Natl Acad. Sci. USA 97, 7446–7451 (2000).
Hauben, E. et al. Passive or active immunization with myelin basic protein promotes recovery from spinal cord contusion. J. Neurosci. 20, 6421–6430 (2000).
Yoles, E. et al. Protective autoimmunity is a physiological response to CNS trauma. J. Neurosci. 21, 3740–3748 (2001).
Urra, X., Miro, F., Chamorro, A. & Planas, A. M. Antigen-specific immune reactions to ischemic stroke. Front. Cell. Neurosci. 8, 278 (2014).
Becker, K. J. et al. Autoimmune responses to the brain after stroke are associated with worse outcome. Stroke 42, 2763–2769 (2011).
Zierath, D. et al. CNS immune responses following experimental stroke. Neurocrit. Care 12, 274–284 (2010).
Planas, A. M. et al. Brain-derived antigens in lymphoid tissue of patients with acute stroke. J. Immunol. 188, 2156–2163 (2012).
Gee, J. M., Kalil, A., Thullbery, M. & Becker, K. J. Induction of immunologic tolerance to myelin basic protein prevents central nervous system autoimmunity and improves outcome after stroke. Stroke 39, 1575–1582 (2008).
Seong, S. Y. & Matzinger, P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 4, 469–478 (2004).
Josefowicz, S. Z., Lu, L. F. & Rudensky, A. Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012).
Liesz, A. et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 15, 192–199 (2009).
Kleinschnitz, C. et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood 121, 679–691 (2013).
Walsh, J. T. et al. Regulatory T cells in central nervous system injury: a double-edged sword. J. Immunol. 193, 5013–5022 (2014).
Walsh, J. T. & Kipnis, J. Regulatory T cells in CNS injury: the simple, the complex and the confused. Trends Mol. Med. 17, 541–547 (2011).
Iadecola, C. & Anrather, J. The immunology of stroke: from mechanisms to translation. Nat. Med. 17, 796–808 (2011).
Gadani, S. P., Walsh, J. T., Smirnov, I., Zheng, J. & Kipnis, J. The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron 85, 703–709 (2015).
Brynskikh, A., Warren, T., Zhu, J. & Kipnis, J. Adaptive immunity affects learning behavior in mice. Brain Behav. Immun. 22, 861–869 (2008).
Kipnis, J., Cohen, H., Cardon, M., Ziv, Y. & Schwartz, M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc. Natl Acad. Sci. USA 101, 8180–8185 (2004).
Radjavi, A., Smirnov, I. & Kipnis, J. Brain antigen-reactive CD4+ T cells are sufficient to support learning behavior in mice with limited T cell repertoire. Brain Behav. Immun. 35, 58–63 (2014).
Filiano, A. J. et al. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature 535, 425–429 (2016).
Moy, S. S. et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 3, 287–302 (2004).
Cohen, H. et al. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. J. Neurobiol. 66, 552–563 (2006).
Quinnies, K. M., Cox, K. H. & Rissman, E. F. Immune deficiency influences juvenile social behavior and maternal behavior. Behav. Neurosci. 129, 331–338 (2015).
Rattazzi, L. et al. CD4+ but not CD8+ T cells revert the impaired emotional behavior of immunocompromised RAG-1-deficient mice. Transl Psychiatry 3, e280 (2013).
Derecki, N. C. et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 207, 1067–1080 (2010).
Wolf, S. A. et al. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J. Immunol. 182, 3979–3984 (2009).
Radjavi, A., Smirnov, I., Derecki, N. & Kipnis, J. Dynamics of the meningeal CD4+ T-cell repertoire are defined by the cervical lymph nodes and facilitate cognitive task performance in mice. Mol. Psychiatry 19, 531–533 (2014).
Balschun, D. et al. Interleukin-6: a cytokine to forget. FASEB J. 18, 1788–1790 (2004).
Supekar, K. et al. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep. 5, 738–747 (2013).
Yizhar, O. et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178 (2011).
Prieto, J. J., Peterson, B. A. & Winer, J. A. Morphology and spatial distribution of GABAergic neurons in cat primary auditory cortex (AI). J. Comp. Neurol. 344, 349–382 (1994).
Clark, S. M. et al. Reduced kynurenine pathway metabolism and cytokine expression in the prefrontal cortex of depressed individuals. J. Psychiatry Neurosci. 41, 386–394 (2016).
Dahl, J. et al. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology 45, 77–86 (2014).
Gupta, S., Aggarwal, S., Rashanravan, B. & Lee, T. Th1- and Th2-like cytokines in CD4+ and CD8+ T cells in autism. J. Neuroimmunol. 85, 106–109 (1998).
Li, X. et al. Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 207, 111–116 (2009).
Masi, A. et al. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol. Psychiatry 20, 440–446 (2015).
Saresella, M. et al. An autistic endophenotype results in complex immune dysfunction in healthy siblings of autistic children. Biol. Psychiatry 66, 978–984 (2009).
Heo, Y., Zhang, Y., Gao, D., Miller, V. M. & Lawrence, D. A. Aberrant immune responses in a mouse with behavioral disorders. PLoS ONE 6, e20912 (2011).
Careaga, M. et al. Immune endophenotypes in children with autism spectrum disorder. Biol. Psychiatry 81, 434–441 (2015).
Zhu, P. J. et al. Suppression of PKR promotes network excitability and enhanced cognition by interferon-γ-mediated disinhibition. Cell 147, 1384–1396 (2011).
Schroder, K., Hertzog, P. J., Ravasi, T. & Hume, D. A. Interferon-γ: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75, 163–189 (2004).
Hu, X. et al. Sensitization of IFN-γ Jak-STAT signaling during macrophage activation. Nat. Immunol. 3, 859–866 (2002).
Lemoine, R. et al. Interferon gamma licensing of human dendritic cells in T-helper-independent CD8+ alloimmunity. Blood 116, 3089–3098 (2010).
Bhat, R. et al. Inhibitory role for GABA in autoimmune inflammation. Proc. Natl Acad. Sci. USA 107, 2580–2585 (2010).
Kerschensteiner, M. et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J. Exp. Med. 189, 865–870 (1999).
Leon-Ponte, M., Ahern, G. P. & O'Connell, P. J. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood 109, 3139–3146 (2007).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl Med. 4, 147ra111 (2012).
Konsman, J. P., Tridon, V. & Dantzer, R. Diffusion and action of intracerebroventricularly injected interleukin-1 in the CNS. Neuroscience 101, 957–967 (2000).
Vitkovic, L. et al. Cytokine signals propagate through the brain. Mol. Psychiatry 5, 604–615 (2000).
Belkaid, Y. & Hand, T. W. Role of the microbiota in immunity and inflammation. Cell 157, 121–141 (2014).
Dinan, T. G. & Cryan, J. F. The impact of gut microbiota on brain and behaviour: implications for psychiatry. Curr. Opin. Clin. Nutr. Metab. Care 18, 552–558 (2015).
Kelley, K. W. et al. Cytokine-induced sickness behavior. Brain Behav. Immun. 17 (Suppl. 1), S112–S118 (2003).
Bluthé, R. M., Michaud, B., Poli, V. & Dantzer, R. Role of IL-6 in cytokine-induced sickness behavior: a study with IL-6 deficient mice. Physiol. Behav. 70, 367–373 (2000).
Bluthé, R. M. et al. Synergy between tumor necrosis factor alpha and interleukin-1 in the induction of sickness behavior in mice. Psychoneuroendocrinology 19, 197–207 (1994).
Blank, T. et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity 44, 901–912 (2016).
Quan, N., Stern, E. L., Whiteside, M. B. & Herkenham, M. Induction of pro-inflammatory cytokine mRNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. J. Neuroimmunol. 93, 72–80 (1999).
Tonelli, L. H. & Postolache, T. T. Tumor necrosis factor alpha, interleukin-1 beta, interleukin-6 and major histocompatibility complex molecules in the normal brain and after peripheral immune challenge. Neurol. Res. 27, 679–684 (2005).
van Dam, A. M., Brouns, M., Louisse, S. & Berkenbosch, F. Appearance of interleukin-1 in macrophages and in ramified microglia in the brain of endotoxin-treated rats: a pathway for the induction of non-specific symptoms of sickness? Brain Res. 588, 291–296 (1992).
Bluthe, R. M., Dantzer, R. & Kelley, K. W. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res. 573, 318–320 (1992).
Hart, B. L. Biological basis of the behavior of sick animals. Neurosci. Biobehav Rev. 12, 123–137 (1988).
Shakhar, K. & Shakhar, G. Why do we feel sick when infected — can altruism play a role? PLoS Biol. 13, e1002276 (2015).
Bos, N., Lefevre, T., Jensen, A. B. & d'Ettorre, P. Sick ants become unsociable. J. Evol. Biol. 25, 342–351 (2012).
Kazlauskas, N., Klappenbach, M., Depino, A. M. & Locatelli, F. F. Sickness behavior in honey bees. Front. Physiol. 7, 261 (2016).
Klein, S. L. Parasite manipulation of the proximate mechanisms that mediate social behavior in vertebrates. Physiol. Behav. 79, 441–449 (2003).
Reiber, C. et al. Change in human social behavior in response to a common vaccine. Ann. Epidemiol. 20, 729–733 (2010).
Campbell, I. L., Hofer, M. J. & Pagenstecher, A. Transgenic models for cytokine-induced neurological disease. Biochim. Biophys. Acta 1802, 903–917 (2010).
Ottum, P. A., Arellano, G., Reyes, L. I., Iruretagoyena, M. & Naves, R. Opposing roles of interferon-gamma on cells of the central nervous system in autoimmune neuroinflammation. Front. Immunol. 6, 539 (2015).
Chesler, D. A. & Reiss, C. S. The role of IFN-γ in immune responses to viral infections of the central nervous system. Cytokine Growth Factor Rev. 13, 441–454 (2002).
Boeke, J. D. & Stoye, J. P. in Retroviruses (eds Coffin, J. M., Hughes, S. H. & Varmus, H. E.) (Cold Spring Harbor Laboratory Press,1997).
Mouse Genome Sequencing Consortium et al. Initial sequencing and comparative analysis of the mouse genome. Nature 420, 520–562 (2002).
Mi, S. et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403, 785–789 (2000).
Fasching, L. et al. TRIM28 represses transcription of endogenous retroviruses in neural progenitor cells. Cell Rep. 10, 20–28 (2015).
Frank, O. et al. Human endogenous retrovirus expression profiles in samples from brains of patients with schizophrenia and bipolar disorders. J. Virol. 79, 10890–10901 (2005).
Christensen, T. Human endogenous retroviruses in neurologic disease. APMIS 124, 116–126 (2016).
Li, W. et al. Human endogenous retrovirus-K contributes to motor neuron disease. Sci. Transl Med. 7, 307ra153 (2015).
Slokar, G. & Hasler, G. Human endogenous retroviruses as pathogenic factors in the development of schizophrenia. Front. Psychiatry 6, 183 (2015).
Gogvadze, E., Stukacheva, E., Buzdin, A. & Sverdlov, E. Human-specific modulation of transcriptional activity provided by endogenous retroviral insertions. J. Virol. 83, 6098–6105 (2009).
Fort, A. et al. Deep transcriptome profiling of mammalian stem cells supports a regulatory role for retrotransposons in pluripotency maintenance. Nat. Genet. 46, 558–566 (2014).
Macfarlan, T. S. et al. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 487, 57–63 (2012).
Chuong, E. B., Elde, N. C. & Feschotte, C. Regulatory evolution of innate immunity through co-option of endogenous retroviruses. Science 351, 1083–1087 (2016).
Flajnik, M. F. & Kasahara, M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat. Rev. Genet. 11, 47–59 (2010).
Huang, S. et al. Discovery of an active RAG transposon illuminates the origins of V(D)J recombination. Cell 166, 102–114 (2016).
Cooper, M. D. & Herrin, B. R. How did our complex immune system evolve? Nat. Rev. Immunol. 10, 2–3 (2010).
Kohn, G. M., King, A. P., Dohme, R., Meredith, G. R. & West, M. J. In the company of cowbirds, Molothrus ater ater: robust patterns of sociability predict reproductive performance. J. Comp. Psychol. 127, 40–48 (2013).
Davis, M. M. et al. Ligand recognition by αβ T cell receptors. Annu. Rev. Immunol. 16, 523–544 (1998).
Itano, A. A. & Jenkins, M. K. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 4, 733–739 (2003).
O'Shea, J. J. & Paul, W. E. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 327, 1098–1102 (2010).
Curry, A. J. et al. Evidence that cytokine-mediated immune interactions induced by Schistosoma mansoni alter disease outcome in mice concurrently infected with Trichuris muris. J. Exp. Med. 181, 769–774 (1995).
Hunter, C. A., Candolfi, E., Subauste, C., Van Cleave, V. & Remington, J. S. Studies on the role of interleukin-12 in acute murine toxoplasmosis. Immunology 84, 16–20 (1995).
Schlager, C. et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature 530, 349–353 (2016).
Kivisakk, P. et al. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc. Natl Acad. Sci. USA 100, 8389–8394 (2003).
Aspelund, A. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212, 991–999 (2015).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Engelhardt, B. & Ransohoff, R. M. Capture, crawl, cross: the T cell code to breach the blood–brain barriers. Trends Immunol. 33, 579–589 (2012).
Kunkel, E. J. & Butcher, E. C. Chemokines and the tissue-specific migration of lymphocytes. Immunity 16, 1–4 (2002).
Yednock, T. A. et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4β1 integrin. Nature 356, 63–66 (1992).
Bosch, I. & Oehmichen, M. Eosinophilic granulocytes in cerebrospinal fluid: analysis of 94 cerebrospinal fluid specimens and review of the literature. J. Neurol. 219, 93–105 (1978).
Degn, M. et al. Increased prevalence of lymphoid tissue inducer cells in the cerebrospinal fluid of patients with early multiple sclerosis. Mult. Scler. 22, 1013–1020 (2016).
Gadani, S. P., Smirnov, I., Smith, A. T., Overall, C. C. & Kipnis, J. Characterization of meningeal type 2 innate lymphocytes and their response to CNS injury. J. Exp. Med. 214, 285–296 (2016).
Kowarik, M. C. et al. Immune cell subtyping in the cerebrospinal fluid of patients with neurological diseases. J. Neurol. 261, 130–143 (2014).
Laperchia, C. et al. Two-photon microscopy imaging of thy1GFP-M transgenic mice: a novel animal model to investigate brain dendritic cell subsets in vivo. PLoS ONE 8, e56144 (2013).
McGavern, D. B. & Kang, S. S. Illuminating viral infections in the nervous system. Nat. Rev. Immunol. 11, 318–329 (2011).
Reuter, U. et al. Delayed inflammation in rat meninges: implications for migraine pathophysiology. Brain 124, 2490–2502 (2001).
Sayed, B. A., Christy, A. L., Walker, M. E. & Brown, M. A. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: a role for neutrophil recruitment? J. Immunol. 184, 6891–6900 (2010).
Garcia, E., Aguilar-Cevallos, J., Silva-Garcia, R. & Ibarra, A. Cytokine and growth factor activation in vivo and in vitro after spinal cord injury. Mediators Inflamm. 2016, 9476020 (2016).
Acknowledgements
The authors thank S. Smith for editing the manuscript and A. Impagliazzo for helping with the artwork. The authors also thank all the members of the Kipnis laboratory for their insightful comments and enlightening ideas. This work was supported by grants from the US National Institutes of Health (AG034113, NS096967 and MH108156 to J.K., and T32-AI007496 to A.J.F.) and the Hartwell Foundation (to A.J.F.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Filiano, A., Gadani, S. & Kipnis, J. How and why do T cells and their derived cytokines affect the injured and healthy brain?. Nat Rev Neurosci 18, 375–384 (2017). https://doi.org/10.1038/nrn.2017.39
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn.2017.39
This article is cited by
-
The brain cytokine orchestra in multiple sclerosis: from neuroinflammation to synaptopathology
Molecular Brain (2024)
-
Somatosensory cortex and central amygdala regulate neuropathic pain-mediated peripheral immune response via vagal projections to the spleen
Nature Neuroscience (2024)
-
Inflammation as common link to progressive neurological diseases
Archives of Toxicology (2024)
-
Neuroimmunology of Cardiovascular Disease
Current Hypertension Reports (2024)
-
Neuroimaging is the new “spatial omic”: multi-omic approaches to neuro-inflammation and immuno-thrombosis in acute ischemic stroke
Seminars in Immunopathology (2023)