Key Points
-
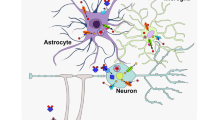
The development of new imaging tools and transgenic animals has greatly improved our understanding of the physiological and pathophysiological role of Zn2+ in brain functioning.
-
Neurons have numerous homeostatic systems to maintain extracellular and intracellular Zn2+ concentrations at levels that are non-toxic. Major systems involved in Zn2+ homeostasis include Zn2+ transporters, Zn2+-importing proteins, metallothioneins, lysosomes and mitochondria.
-
Zn2+ has a major role in controlling synaptic excitability as it can greatly modulate both glutamatergic and GABA (γ-aminobutyric acid)-ergic neurotransmission.
-
Zn2+ is also potently neurotoxic and has an important role in triggering neuronal death in transient global ischaemia and brain trauma.
-
Zn2+ is also instrumental in the development of amyloid plaques in Alzheimer's disease. Pharmacological interventions aimed at restoring Zn2+ homeostasis in the brain are yielding promising results in the treatment of patients with Alzheimer's disease.
Abstract
The past few years have witnessed dramatic progress on all frontiers of zinc neurobiology. The recent development of powerful tools, including zinc-sensitive fluorescent probes, selective chelators and genetically modified animal models, has brought a deeper understanding of the roles of this cation as a crucial intra- and intercellular signalling ion of the CNS, and hence of the neurophysiological importance of zinc-dependent pathways and the injurious effects of zinc dyshomeostasis. The development of some innovative therapeutic strategies is aimed at controlling and preventing the damaging effects of this cation in neurological conditions such as stroke and Alzheimer's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Frederickson, C. J., Koh, J. Y. & Bush, A. I. The neurobiology of zinc in health and disease. Nature Rev. Neurosci. 6, 449–462 (2005).
Shen, H. et al. Zinc distribution and expression pattern of ZnT3 in mouse brain. Biol. Trace Elem. Res. 119, 166–174 (2007).
Linkous, D. H. et al. Evidence that the ZNT3 protein controls the total amount of elemental zinc in synaptic vesicles. J. Histochem. Cytochem. 56, 3–6 (2008).
Sensi, S. L., Yin, H. Z. & Weiss, J. H. AMPA/kainate receptor-triggered Zn2+ entry into cortical neurons induces mitochondrial Zn2+ uptake and persistent mitochondrial dysfunction. Eur. J. Neurosci. 12, 3813–3818 (2000). First study to show that Zn2+ can be sequestered in mitochondria and promote strong mitochondrial dysfunction.
Colvin, R. A., Laskowski, M. & Fontaine, C. P. Zinquin identifies subcellular compartmentalization of zinc in cortical neurons. Relation to the trafficking of zinc and the mitochondrial compartment. Brain Res. 1085, 1–10 (2006).
Hwang, J. J., Lee, S. J., Kim, T. Y., Cho, J. H. & Koh, J. Y. Zinc and 4-hydroxy-2-nonenal mediate lysosomal membrane permeabilization induced by H2O2 in cultured hippocampal neurons. J. Neurosci. 28, 3114–3122 (2008). First study to show that Zn2+ can be sequestered in neuronal lysosomes and trigger autophagy.
Dittmer, P. J., Miranda, J. G., Gorski, J. A. & Palmer, A. E. Genetically encoded sensors to elucidate spatial distribution of cellular zinc. J. Biol. Chem. 284, 16289–16297 (2009).
Smart, T. G., Hosie, A. M. & Miller, P. S. Zn2+ ions: modulators of excitatory and inhibitory synaptic activity. Neuroscientist 10, 432–442 (2004).
Besser, L. et al. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J. Neurosci. 29, 2890–2901 (2009). Study leading to the functional identification of a synaptic Zn2+-sensing receptor that can modulate intracellular Ca2+ signalling.
Tonder, N., Johansen, F. F., Frederickson, C. J., Zimmer, J. & Diemer, N. H. Possible role of zinc in the selective degeneration of dentate hilar neurons after cerebral ischemia in the adult rat. Neurosci. Lett. 109, 247–252 (1990).
Koh, J. Y. et al. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science 272, 1013–1016 (1996). First study to show that Zn2+ has a key role in triggering neuronal death in transient global ischaemia.
Lee, J. Y., Kim, J. H., Palmiter, R. D. & Koh, J. Y. Zinc released from metallothionein-III may contribute to hippocampal CA1 and thalamic neuronal death following acute brain injury. Exp. Neurol. 184, 337–347 (2003).
Lee, J. Y., Cole, T. B., Palmiter, R. D. & Koh, J. Y. Accumulation of zinc in degenerating hippocampal neurons of ZnT3-null mice after seizures: evidence against synaptic vesicle origin. J. Neurosci. 20, RC79 (2000). First study showing injurious intraneuronal Zn2+ release.
Aizenman, E. et al. Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J. Neurochem. 75, 1878–1888 (2000). First study demonstrating that oxidative stress can promote [Zn2+]i rises of intracellular origin that are sufficient to elicit neuronal death.
Ohana, E. et al. Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J. Biol. Chem. 284, 17677–17686 (2009).
Chao, Y. & Fu, D. Kinetic study of the antiport mechanism of an Escherichia coli zinc transporter, ZitB. J. Biol. Chem. 279, 12043–12050 (2004).
MacDiarmid, C. W., Milanick, M. A. & Eide, D. J. Biochemical properties of vacuolar zinc transport systems of Saccharomyces cerevisiae. J. Biol. Chem. 277, 39187–39194 (2002).
Lu, M. & Fu, D. Structure of the zinc transporter YiiP. Science 317, 1746–1748 (2007).
Lipton, P. Ischemic cell death in brain neurons. Physiol. Rev. 79, 1431–1568 (1999).
Palmiter, R. D., Cole, T. B., Quaife, C. J. & Findley, S. D. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl Acad. Sci. USA 93, 14934–14939 (1996). First demonstration of the role of ZnT3 at excitatory synapses.
Ruiz, A., Walker, M. C., Fabian-Fine, R. & Kullmann, D. M. Endogenous zinc inhibits GABAA receptors in a hippocampal pathway. J. Neurophysiol. 91, 1091–1096 (2004).
Wang, Z., Danscher, G., Kim, Y. K., Dahlstrom, A. & Mook, J. S. Inhibitory zinc-enriched terminals in the mouse cerebellum: double-immunohistochemistry for zinc transporter 3 and glutamate decarboxylase. Neurosci. Lett. 321, 37–40 (2002).
Lopantsev, V., Wenzel, H. J., Cole, T. B., Palmiter, R. D. & Schwartzkroin, P. A. Lack of vesicular zinc in mossy fibers does not affect synaptic excitability of CA3 pyramidal cells in zinc transporter 3 knockout mice. Neuroscience 116, 237–248 (2003).
Ismail, T., Mauerhofer, E. & Slomianka, L. The hippocampal region of rats and mice after a single i.p. dose of clioquinol: loss of synaptic zinc, cell death and c-Fos induction. Neuroscience 157, 697–707 (2008).
Blasco-Ibanez, J. M. et al. Chelation of synaptic zinc induces overexcitation in the hilar mossy cells of the rat hippocampus. Neurosci. Lett. 355, 101–104 (2004).
Takeda, A., Hirate, M., Tamano, H., Nisibaba, D. & Oku, N. Susceptibility to kainate-induced seizures under dietary zinc deficiency. J. Neurochem. 85, 1575–1580 (2003).
Doering, P. et al. Changes in the vesicular zinc pattern following traumatic brain injury. Neuroscience 150, 93–103 (2007).
Palmiter, R. D. & Findley, S. D. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 14, 639–649 (1995).
Nolte, C. et al. ZnT-1 expression in astroglial cells protects against zinc toxicity and slows the accumulation of intracellular zinc. Glia 48, 145–155 (2004).
Tsuda, M. et al. Expression of zinc transporter gene, ZnT-1, is induced after transient forebrain ischemia in the gerbil. J. Neurosci. 17, 6678–6684 (1997).
Aguilar-Alonso, P. et al. The increase in zinc levels and upregulation of zinc transporters are mediated by nitric oxide in the cerebral cortex after transient ischemia in the rat. Brain Res. 1200, 89–98 (2008).
Ohana, E. et al. Silencing of ZnT-1 expression enhances heavy metal influx and toxicity. J. Mol. Med. 84, 753–763 (2006).
Qin, Y., Thomas, D., Fontaine, C. P. & Colvin, R. A. Silencing of ZnT1 reduces Zn2+ efflux in cultured cortical neurons. Neurosci. Lett. 450, 206–210 (2009).
Segal, D. et al. A role for ZnT-1 in regulating cellular cation influx. Biochem. Biophys. Res. Commun. 323, 1145–1150 (2004).
Sensi, S. L. et al. Measurement of intracellular free zinc in living cortical neurons: routes of entry. J. Neurosci. 17, 9554–9564 (1997).
Ohana, E. et al. A sodium zinc exchange mechanism is mediating extrusion of zinc in mammalian cells. J. Biol. Chem. 279, 4278–4284 (2004).
Qin, Y., Thomas, D., Fontaine, C. P. & Colvin, R. A. Mechanisms of Zn2+ efflux in cultured cortical neurons. J. Neurochem. 107, 1304–1313 (2008).
Gaither, L. A. & Eide, D. J. Functional expression of the human hZIP2 zinc transporter. J. Biol. Chem. 275, 5560–5564 (2000).
Wang, F. et al. Zinc-stimulated endocytosis controls activity of the mouse ZIP1 and ZIP3 zinc uptake transporters. J. Biol. Chem. 279, 24631–24639 (2004).
Dufner-Beattie, J., Kuo, Y. M., Gitschier, J. & Andrews, G. K. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5. J. Biol. Chem. 279, 49082–49090 (2004).
Kambe, T. & Andrews, G. K. Novel proteolytic processing of the ectodomain of the zinc transporter ZIP4 (SLC39A4) during zinc deficiency is inhibited by acrodermatitis enteropathica mutations. Mol. Cell. Biol. 29, 129–139 (2009).
Dufner-Beattie, J., Huang, Z. L., Geiser, J., Xu, W. & Andrews, G. K. Generation and characterization of mice lacking the zinc uptake transporter ZIP3. Mol. Cell. Biol. 25, 5607–5615 (2005).
Paoletti, P., Vergnano, A. M., Barbour, B. & Casado, M. Zinc at glutamatergic synapses. Neuroscience 158, 126–136 (2009).
Paoletti, P., Ascher, P. & Neyton, J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J. Neurosci. 17, 5711–5725 (1997). First characterization of a high-affinity site for Zn2+ that controls NMDA receptor inhibition.
Rachline, J., Perin-Dureau, F., Le Goff, A., Neyton, J. & Paoletti, P. The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J. Neurosci. 25, 308–317 (2005).
Hirzel, K. et al. Hyperekplexia phenotype of glycine receptor α1 subunit mutant mice identifies Zn2+ as an essential endogenous modulator of glycinergic neurotransmission. Neuron 52, 679–690 (2006).
Qian, J. & Noebels, J. L. Visualization of transmitter release with zinc fluorescence detection at the mouse hippocampal mossy fibre synapse. J. Physiol. 566, 747–758 (2005).
Qian, J. & Noebels, J. L. Exocytosis of vesicular zinc reveals persistent depression of neurotransmitter release during metabotropic glutamate receptor long-term depression at the hippocampal CA3-CA1 synapse. J. Neurosci. 26, 6089–6095 (2006). Study implicating Zn2+ in the modulation of long-term depression.
Kodirov, S. A. et al. Synaptically released zinc gates long-term potentiation in fear conditioning pathways. Proc. Natl Acad. Sci. USA 103, 15218–15223 (2006).
Huang, Y. Z., Pan, E., Xiong, Z. Q. & McNamara, J. O. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron 57, 546–558 (2008). First study demonstrating that Zn2+ can transactivate the TrkB receptor.
Kay, A. R., Neyton, J. & Paoletti, P. A startling role for synaptic zinc. Neuron 52, 572–574 (2006).
Attwell, D., Barbour, B. & Szatkowski, M. Nonvesicular release of neurotransmitter. Neuron 11, 401–407 (1993).
Vogt, K., Mellor, J., Tong, G. & Nicoll, R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron 26, 187–196 (2000).
Molnar, P. & Nadler, J. V. Synaptically-released zinc inhibits N-methyl-D-aspartate receptor activation at recurrent mossy fiber synapses. Brain Res. 910, 205–207 (2001).
Mott, D. D., Benveniste, M. & Dingledine, R. J. pH-dependent inhibition of kainate receptors by zinc. J. Neurosci. 28, 1659–1671 (2008). First study demonstrating that Zn2+ can inhibit kainate receptors.
Sindreu, C. B., Varoqui, H., Erickson, J. D. & Perez-Clausell, J. Boutons containing vesicular zinc define a subpopulation of synapses with low AMPAR content in rat hippocampus. Cereb. Cortex 13, 823–829 (2003).
Nakashima, A. S. & Dyck, R. H. Zinc and cortical plasticity. Brain Res. Rev. 59, 347–373 (2009).
Hwang, J. J., Park, M. H., Choi, S. Y. & Koh, J. Y. Activation of the Trk signaling pathway by extracellular zinc: role of metalloproteinases. J. Biol. Chem. 280, 11995–12001 (2005). First study demonstrating that Zn2+ can promote the maturation of pro-BDNF to BDNF and therefore indirectly activate TrkB-dependent signalling.
Smith, R. M. NIST critically selected stability constants of metal complexes: version 8. NIST Scientific and Technical Databases [online], (2009).
Stork, C. J. & Li, Y. V. Intracellular zinc elevation measured with a “calcium-specific” indicator during ischemia and reperfusion in rat hippocampus: a question on calcium overload. J. Neurosci. 26, 10430–10437 (2006). Study showing that most of the OGD-driven changes of Ca2+-sensitive probes depends on Zn2+.
Hofer, A. M., Fasolato, C. & Pozzan, T. Capacitative Ca2+ entry is closely linked to the filling state of internal Ca2+ stores: a study using simultaneous measurements of ICRAC and intraluminal [Ca2+]. J. Cell Biol. 140, 325–334 (1998).
Arslan, P., Di Virgilio, F., Beltrame, M., Tsien, R. Y. & Pozzan, T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J. Biol. Chem. 260, 2719–2727 (1985).
Medvedeva, Y. V., Lin, B., Shuttleworth, C. W. & Weiss, J. H. Intracellular Zn2+ accumulation contributes to synaptic failure, mitochondrial depolarization, and cell death in an acute slice oxygen–glucose deprivation model of ischemia. J. Neurosci. 29, 1105–1114 (2009). Study showing the synergy between Ca2+ and Zn2+ in mediating ischaemic neuronal death.
Hyrc, K., Handran, S. D., Rothman, S. M. & Goldberg, M. P. Ionized intracellular calcium concentration predicts excitotoxic neuronal death: observations with low-affinity fluorescent calcium indicators. J. Neurosci. 17, 6669–6677 (1997).
Jiang, D., Sullivan, P. G., Sensi, S. L., Steward, O. & Weiss, J. H. Zn2+ induces permeability transition pore opening and release of pro-apoptotic peptides from neuronal mitochondria. J. Biol. Chem. 276, 47524–47529 (2001).
Bossy-Wetzel, E. et al. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron 41, 351–365 (2004).
Galluzzi, L., Blomgren, K. & Kroemer, G. Mitochondrial membrane permeabilization in neuronal injury. Nature Rev. Neurosci. 10, 481–494 (2009).
Lee, J. M. et al. Zinc translocation accelerates infarction after mild transient focal ischemia. Neuroscience 115, 871–878 (2002).
Yin, H. Z., Sensi, S. L., Ogoshi, F. & Weiss, J. H. Blockade of Ca2+-permeable AMPA/kainate channels decreases oxygen-glucose deprivation-induced Zn2+ accumulation and neuronal loss in hippocampal pyramidal neurons. J. Neurosci. 22, 1273–1279 (2002).
Noh, K. M. et al. Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against global ischemia-induced death. Proc. Natl. Acad. Sci. USA 102, 12230–12235 (2005). Study indicating the importance of AMPAR Ca–Zn pharmacological blockade in the context of TGI.
Malaiyandi, L. M., Dineley, K. E. & Reynolds, I. J. Metallothionein overexpression enhances oxidant-induced zinc release in astrocytes. Soc. Neurosci. Abstr. 27, 868.16 (2001).
Malaiyandi, L. M., Vergun, O., Dineley, K. E. & Reynolds, I. J. Direct visualization of mitochondrial zinc accumulation reveals uniporter-dependent and -independent transport mechanisms. J. Neurochem. 93, 1242–1250 (2005).
Sensi, S. L. et al. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc. Natl Acad. Sci. USA 100, 6157–6162 (2003).
Pal, S., Hartnett, K. A., Nerbonne, J. M., Levitan, E. S. & Aizenman, E. Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels. J. Neurosci. 23, 4798–4802 (2003).
Hershfinkel, M. et al. Intracellular zinc inhibits KCC2 transporter activity. Nature Neurosci. 12, 725–727 (2009).
Gazaryan, I. G., Krasinskaya, I. P., Kristal, B. S. & Brown, A. M. Zinc irreversibly damages major enzymes of energy production and antioxidant defense prior to mitochondrial permeability transition. J. Biol. Chem. 282, 24373–24380 (2007).
Dineley, K. E., Richards, L. L., Votyakova, T. V. & Reynolds, I. J. Zinc causes loss of membrane potential and elevates reactive oxygen species in rat brain mitochondria. Mitochondrion 5, 55–65 (2005).
Sensi, S. L., Yin, H. Z., Carriedo, S. G., Rao, S. S. & Weiss, J. H. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc. Natl Acad. Sci. USA 96, 2414–2419 (1999).
Dineley, K. E., Votyakova, T. V. & Reynolds, I. J. Zinc inhibition of cellular energy production: implications for mitochondria and neurodegeneration. J. Neurochem. 85, 563–570 (2003).
Bonanni, L. et al. Zinc-dependent multi-conductance channel activity in mitochondria isolated from ischemic brain. J. Neurosci. 26, 6851–6862 (2006).
Malaiyandi, L. M., Honick, A. S., Rintoul, G. L., Wang, Q. J. & Reynolds, I. J. Zn2+ inhibits mitochondrial movement in neurons by phosphatidylinositol 3-kinase activation. J. Neurosci. 25, 9507–9514 (2005).
Noh, K. M., Kim, Y. H. & Koh, J. Y. Mediation by membrane protein kinase C of zinc-induced oxidative neuronal injury in mouse cortical cultures. J. Neurochem. 72, 1609–1616 (1999).
Kim, Y. H. & Koh, J. Y. The role of NADPH oxidase and neuronal nitric oxide synthase in zinc-induced poly(ADP-ribose) polymerase activation and cell death in cortical culture. Exp. Neurol. 177, 407–418 (2002).
Sheline, C. T., Behrens, M. M. & Choi, D. W. Zinc-induced cortical neuronal death: contribution of energy failure attributable to loss of NAD+ and inhibition of glycolysis. J. Neurosci. 20, 3139–3146 (2000).
Lee, J. Y., Kim, Y. H. & Koh, J. Y. Protection by pyruvate against transient forebrain ischemia in rats. J. Neurosci. 21, RC171 (2001).
Imai, S., Armstrong, C. M., Kaeberlein, M. & Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 (2000).
Cai, A. L., Zipfel, G. J. & Sheline, C. T. Zinc neurotoxicity is dependent on intracellular NAD levels and the sirtuin pathway. Eur. J. Neurosci. 24, 2169–2176 (2006).
Suh, S. W. et al. Sequential release of nitric oxide, zinc, and superoxide in hypoglycemic neuronal death. J. Cereb. Blood Flow Metab. 28, 1697–1706 (2008).
Lee, S. J., Cho, K. S. & Koh, J. Y. Oxidative injury triggers autophagy in astrocytes: the role of endogenous zinc. Glia 57, 1351–1361 (2009).
Hoyer-Hansen, M. & Jaattela, M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 14, 1576–1582 (2007).
Kerchner, G. A., Canzoniero, L. M., Yu, S. P., Ling, C. & Choi, D. W. Zn2+ current is mediated by voltage-gated Ca2+ channels and enhanced by extracellular acidity in mouse cortical neurones. J. Physiol. 528, 39–52 (2000).
Jeng, J.-M., Jia, Y., Bonanni, L. & Weiss, J. H. Divergent effects of pH on Zn2+ and Ca2+ flux through Ca2+-permeable AMPA/kainate channels (CAKR). Soc. Neurosci. Abstr. 539.9. (2002).
Traynelis, S. & Cull-Candy, S. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature 345, 347–350 (1990).
Frazzini, V. et al. Mild acidosis enhances AMPA receptor-mediated intracellular zinc mobilization in cortical neurons. Mol. Med. 13, 356–361 (2007).
Xiong, Z. G. et al. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118, 687–698 (2004).
Wang, W. Z. et al. Modulation of acid-sensing ion channel currents, acid-induced increase of intracellular Ca2+, and acidosis-mediated neuronal injury by intracellular pH. J. Biol. Chem. 281, 29369–29378 (2006).
Hey, J. G., Chu, X. P., Seeds, J., Simon, R. P. & Xiong, Z. G. Extracellular zinc protects against acidosis-induced injury of cells expressing Ca2+-permeable acid-sensing ion channels. Stroke 38, 670–673 (2007).
Dineley, K. E., Brocard, J. B. & Reynolds, I. J. Elevated intracellular zinc and altered proton homeostasis in forebrain neurons. Neuroscience 114, 439–449 (2002).
Park, J. A., Lee, J. Y., Sato, T. A. & Koh, J. Y. Co-induction of p75NTR and p75NTR-associated death executor in neurons after zinc exposure in cortical culture or transient ischemia in the rat. J. Neurosci. 20, 9096–9103 (2000).
Park, J. A. & Koh, J. Y. Induction of an immediate early gene egr-1 by zinc through extracellular signal-regulated kinase activation in cortical culture: its role in zinc-induced neuronal death. J. Neurochem. 73, 450–456 (1999).
Lee, J. Y., Kim, Y. J., Kim, T. Y., Koh, J. Y. & Kim, Y. H. Essential role for zinc-triggered p75NTR activation in preconditioning neuroprotection. J. Neurosci. 28, 10919–10927 (2008).
Nowak, G. et al. Zinc treatment induces cortical brain-derived neurotrophic factor gene expression. Eur. J. Pharmacol. 492, 57–59 (2004).
Bush, A. I. et al. Rapid induction of Alzheimer Aβ amyloid formation by zinc. Science 265, 1464–1467 (1994). First study to identify the pro-aggregant activity of Zn2+.
Bush, A. I., Pettingell, W. H. Jr, Paradis, M. D. & Tanzi, R. E. Modulation of Aβ adhesiveness and secretase site cleavage by zinc. J. Biol. Chem. 269, 12152–12158 (1994).
Ha, C., Ryu, J. & Park, C. B. Metal ions differentially influence the aggregation and deposition of Alzheimer's β-amyloid on a solid template. Biochemistry 46, 6118–6125 (2007).
Noy, D. et al. Zinc-amyloid β interactions on a millisecond time-scale stabilize non-fibrillar Alzheimer-related species. J. Am. Chem. Soc. 130, 1376–1383 (2008).
Huang, X. et al. The Aβ peptide of Alzheimer's Disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry 38, 7609–7616 (1999).
Rottkamp, C. et al. Redox-active iron mediates amyloid-β toxicity. Free Radic. Biol. Med. 30, 447–450 (2001).
Opazo, C. et al. Metalloenzyme-like activity of Alzheimer's disease β-amyloid. Cu-dependent catalytic conversion of dopamine, cholesterol, and biological reducing agents to neurotoxic H2O2 . J. Biol. Chem. 277, 40302–40308 (2002).
Dukes, K. D., Rodenberg, C. F. & Lammi, R. K. Monitoring the earliest amyloid-β oligomers via quantized photobleaching of dye-labeled peptides. Anal. Biochem. 382, 29–34 (2008).
Lesne, S. et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature 440, 352–357 (2006).
Garai, K., Sengupta, P., Sahoo, B. & Maiti, S. Selective destabilization of soluble amyloid β oligomers by divalent metal ions. Biochem. Biophys. Res. Commun. 345, 210–215 (2006).
Lee, J. Y., Cole, T. B., Palmiter, R. D., Suh, S. W. & Koh, J. Y. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc. Natl Acad. Sci. USA 99, 7705–7710 (2002).
Lee, J.-Y. et al. Estrogen decreases zinc transporter 3 expression and synaptic vesicle zinc levels in mouse brain. J. Biol. Chem. 279, 8602–8607 (2004).
Friedlich, A. L. et al. Neuronal zinc exchange with the blood vessel wall promotes cerebral amyloid angiopathy in an animal model of Alzheimer's disease. J. Neurosci. 24, 3453–3459 (2004).
Smith, J. L., Xiong, S., Markesbery, W. R. & Lovell, M. A. Altered expression of zinc transporters-4 and -6 in mild cognitive impairment, early and late Alzheimer's disease brain. Neuroscience 140, 879–888 (2006).
Zhang, L. H. et al. Altered expression and distribution of zinc transporters in APP/PS1 transgenic mouse brain. Neurobiol. Aging 28 Mar 2008 (doi:10.1016/j.neurobiolaging.2008.02.018).
Deshpande, A., Kawai, H., Metherate, R., Glabe, C. G. & Busciglio, J. A role for synaptic zinc in activity-dependent Aβ oligomer formation and accumulation at excitatory synapses. J. Neurosci. 29, 4004–4015 (2009).
Stanika, R. I. et al. Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proc. Natl Acad. Sci. USA 106, 9854–9859 (2009).
Uchida, Y., Gomi, F., Masumizu, T. & Miura, Y. Growth inhibitory factor prevents neurite extension and the death of cortical neurons caused by high oxygen exposure through hydroxyl radical scavenging. J. Biol. Chem. 277, 32353–32359 (2002).
Meloni, G. et al. Metal swap between Zn7-metallothionein-3 and amyloid-β-Cu protects against amyloid-β toxicity. Nature Chem. Biol. 4, 366–372 (2008).
Sensi, S. L., Rapposelli, I. G., Frazzini, V. & Mascetra, N. Altered oxidant-mediated intraneuronal zinc mobilization in a triple transgenic mouse model of Alzheimer's disease. Exp. Gerontol. 43, 488–492 (2008).
Opazo, C. et al. Metalloenzyme-like activity of Alzheimer's disease β-amyloid: Cu-dependent catalytic conversion of dopamine, cholesterol and biological reducing agents to neurotoxic H2O2 . J. Biol. Chem. 277, 40302–40308 (2002).
Howell, G. A., Welch, M. G. & Frederickson, C. J. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature 308, 736–738 (1984).
Bush, A. I. Drug development based on the metals hypothesis of Alzheimer's disease. J. Alzheimers Dis. 15, 223–240 (2008).
Strozyk, D. et al. Zinc and copper modulate Alzheimer Aβ levels in human cerebrospinal fluid. Neurobiol. Aging 30, 1069–1077 (2009).
Danscher, G. et al. Increased amount of zinc in the hippocampus and amygdala of Alzheimer's diseased brains: a proton-induced X-ray emission spectroscopic analysis of cryostat sections from autopsy material. J. Neurosci. Methods 76, 53–59 (1997).
Lovell, M. A., Robertson, J. D., Teesdale, W. J., Campbell, J. L. & Markesbery, W. R. Copper, iron and zinc in Alzheimer's disease senile plaques. J. Neurol. Sci. 158, 47–52 (1998).
Opazo, C. et al. Radioiodinated clioquinol as a biomarker for β-amyloid: Zn2+ complexes in Alzheimer's disease. Aging Cell 5, 69–79 (2006).
Religa, D. et al. Elevated cortical zinc in Alzheimer disease. Neurology 67, 69–75 (2006).
Smith, J. L., Xiong, S. & Lovell, M. A. 4-Hydroxynonenal disrupts zinc export in primary rat cortical cells. Neurotoxicology 27, 1–5 (2006).
Stoltenberg, M. et al. Amyloid plaques arise from zinc-enriched cortical layers in APP/PS1 transgenic mice and are paradoxically enlarged with dietary zinc deficiency. Neuroscience 150, 357–369 (2007).
Takeda, A., Minami, A., Takefuta, S., Tochigi, M. & Oku, N. Zinc homeostasis in the brain of adult rats fed zinc-deficient diet. J. Neurosci. Res. 63, 447–452 (2001).
Chowanadisai, W., Kelleher, S. L. & Lonnerdal, B. Zinc deficiency is associated with increased brain zinc import and LIV-1 expression and decreased ZnT-1 expression in neonatal rats. J. Nutr. 135, 1002–1007 (2005).
Lovell, M. A. A potential role for alterations of zinc and zinc transport proteins in the progression of Alzheimer's disease. J. Alzheimers Dis. 16, 471–483 (2009).
Bjorkdahl, C., Sjogren, M. J., Winblad, B. & Pei, J. J. Zinc induces neurofilament phosphorylation independent of p70 S6 kinase in N2a cells. Neuroreport 16, 591–595 (2005).
Suh, S. W. et al. Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer's diseased brains. Brain Res. 852, 274–278 (2000).
Cherny, R. A. et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits β-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron 30, 665–676 (2001). Study indicating that restoring Cu2+ and Zn2+ homeostasis in the brain can dramatically reduce the amyloid load.
Maynard, C. J. et al. Overexpression of Alzheimer's disease β-amyloid opposes the age-dependent elevations of brain copper and iron levels. J. Biol. Chem. 277, 44670–44676 (2002).
Maynard, C. J. et al. Gender and genetic background effects on brain metal levels in APP transgenic and normal mice: implications for Alzheimer β-amyloid pathology. J. Inorg. Biochem. 100, 952–962 (2006).
Adlard, P. A. et al. Rapid restoration of cognition in Alzheimer's transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Aβ. Neuron 59, 43–55 (2008).
Ritchie, C. W. et al. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Aβ amyloid deposition and toxicity in Alzheimer's disease: a pilot phase 2 clinical trial. Arch. Neurol. 60, 1685–1691 (2003).
Colvin, R. A. et al. Insights into Zn2+ homeostasis in neurons from experimental and modeling studies. Am. J. Physiol. Cell Physiol. 294, 726–742 (2008).
Lannfelt, L. et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Aβ as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 7, 779–786 (2008).
Lee, J. Y., Friedman, J. E., Angel, I., Kozak, A. & Koh, J. Y. The lipophilic metal chelator DP-109 reduces amyloid pathology in brains of human beta-amyloid precursor protein transgenic mice. Neurobiol. Aging 25, 1315–1321 (2004).
Turrigiano, G. G. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135, 422–435 (2008).
Assaf, S. Y. & Chung, S. H. Release of endogenous Zn2+ from brain tissue during activity. Nature 308, 734–736 (1984).
Kay, A. R. Imaging synaptic zinc: promises and perils. Trends Neurosci. 29, 200–206 (2006).
Atar, D., Backx, P. H., Appel, M. M., Gao, W. D. & Marban, E. Excitation-transcription coupling mediated by zinc influx through voltage-dependent calcium channels. J. Biol. Chem. 270, 2473–2477 (1995).
Caporale, T. et al. Ratiometric-pericam-mt, a novel tool to evaluate intramitochondrial zinc. Exp. Neurol. 218, 228–234 (2009).
Feng, W. & Zhang, M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density. Nature Rev. Neurosci. 10, 87–99 (2009).
Baron, M. K. et al. An architectural framework that may lie at the core of the postsynaptic density. Science 311, 531–535 (2006).
Gundelfinger, E. D., Boeckers, T. M., Baron, M. K. & Bowie, J. U. A role for zinc in postsynaptic density asSAMbly and plasticity? Trends Biochem. Sci. 31, 366–373 (2006).
Acknowledgements
We are in debt to M. Hershfinkel, A. Vergnano, V. Frazzini, and M.E. Oberschlake for help with the preparation of the manuscript. S.L.S. is supported by funds from the Italian Department of Education (FIRB 2003; PRIN 2006). P.P. is supported by Institut National de la Santé et de la Recherche Médicale (INSERM), France, Agence Nationale de la Recherche (ANR), France, and Fondation pour la Recherche Médicale (FRM; Equipe FRM grant). A.I.B. is supported with funds from the National Health and Medical Research Council of Australia, and The Australian Research Council. Work described in this review was partially supported by ISF grant # 985/05 and GIF grant # 917-119.1/2006 to I.S.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
A.I.B. is a paid consultant for and shareholder in Prana Biotechnology Ltd. S.L.S. is a shareholder in Prana Biotechnology Ltd.
Related links
Glossary
- Hyperekplexia
-
A pathological condition observed in newborns that is associated with startle responses (forced eye closure with limb and arm extension, followed by a period of generalized hypertonia) to tactile or acoustic stimuli, truncal hypertonia and episodic apnea. Hyperekplexia can be familial or sporadic. The familial form has been linked to mutations in the α-subunit of the glycine receptor.
- Transient global ischaemia
-
A particular form of cerebral ischaemia that follows conditions such as cardiac arrest or the near-drowning syndrome. It is characterized by a delayed (7–10 days after the insult) selective form of neuronal death that preferentially targets the hippocampus (in particular its CA1 subregion). TGI seems to be largely modulated by the activation of a subtype of ionotropic glutamate receptor, the AMPARCa–Zn, that lacks the GluR2 subunit and therefore is highly permeable to Ca2+ and Zn2+.
- Unfolded protein response
-
(UPR). A cellular stress response modulated by the endoplasmic reticulum (ER). Unfolded or misfolded proteins, when accumulated in the ER, activate many chaperones that facilitate proper protein folding in an attempt to restore ER function. When protein folding cannot be restored the UPR has a crucial role in initiating apoptotic pathways that lead to cell death.
- Congophilic angiopathy
-
The pathological accumulation of β-amyloid in the walls of CNS blood vessels (also known as cerebral amyloid angiopathy). Congophilic derives from the fact that such amyloid deposition can be revealed by Congo red staining.
- Amyloid-β42
-
The form of amyloid-β that is most enriched in amyloid pathology and is most prone to aggregation in vitro. It is also overproduced in some forms of familial AD caused by mutations of APP or presenilins.
Rights and permissions
About this article
Cite this article
Sensi, S., Paoletti, P., Bush, A. et al. Zinc in the physiology and pathology of the CNS. Nat Rev Neurosci 10, 780–791 (2009). https://doi.org/10.1038/nrn2734
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn2734
This article is cited by
-
Biometals in Alzheimer disease: emerging therapeutic and diagnostic potential of molybdenum and iodine
Journal of Translational Medicine (2023)
-
Geroprotective interventions in the 3xTg mouse model of Alzheimer’s disease
GeroScience (2023)
-
Selective detection of metal ions, sulfites and glutathione with fluorescent pyrazolines: a review
Environmental Chemistry Letters (2023)
-
Role of TPEN in Amyloid-β25–35-Induced Neuronal Damage Correlating with Recovery of Intracellular Zn2+ and Intracellular Ca2+ Overloading
Molecular Neurobiology (2023)
-
Extracellular Alkalosis Reduces the Neurotoxicity of Zinc Ions in Cultured Cerebellar Granule Neurons
Biological Trace Element Research (2023)