Key Points
-
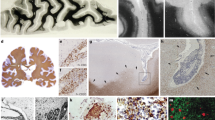
Although multiple sclerosis has been characterized previously as a white matter disease, it is becoming increasingly apparent that extensive cortical and deep grey matter pathology can be present.
-
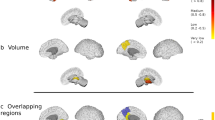
Several imaging studies have documented convincing correlations between white matter lesions and grey matter atrophy, suggesting that neurodegeneration can be a consequence of white matter demyelination via retrograde degeneration.
-
Another equally important theory suggests that white matter and grey matter demyelination are two, at least partly, independent phenomena and that neuronal loss is not caused by white matter abnormalities per se.
-
Several inflammatory cells types, including CD4+ and CD8+ T cells, are implicated in grey matter damage.
-
A role for B cells and meningeal inflammatory infiltrates in multiple sclerosis has been recently proposed.
-
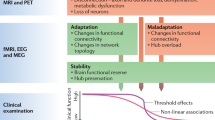
It is becoming more widely accepted that microglial activation is necessary and crucial for host defence and neuronal survival, whereas microglial over-activation may be deleterious to neurons and oligodendrocytes.
-
The neuronal energy deficit is crucial for inducing axonal swelling and subsequent neuronal death, especially when it occurs as a consequence of inflammation. Several lines of evidence have led to the hypothesis that mitochondrial injury, and therefore the energy deficit, is a primary phenomenon in multiple sclerosis.
-
A recent theory — the inside-out model — notes the inconsistencies in the inflammatory model described above and suggests a degenerative model as the primary cause of the disease.
Abstract
Multiple sclerosis is characterized at the gross pathological level by the presence of widespread focal demyelinating lesions of the myelin-rich white matter. However, it is becoming clear that grey matter is not spared, even during the earliest phases of the disease. Furthermore, grey matter damage may have an important role both in physical and cognitive disability. Grey matter pathology involves both inflammatory and neurodegenerative mechanisms, but the relationship between the two is unclear. Histological, immunological and neuroimaging studies have provided new insight in this rapidly expanding field, and form the basis of the most recent hypotheses on the pathogenesis of grey matter damage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Noseworthy, J. H., Lucchinetti, C., Rodriguez, M. & Weinshenker, B. G. Multiple sclerosis. N. Engl. J. Med. 343, 938–952 (2000).
Sospedra, M. & Martin, R. Immunology of multiple sclerosis. Annu. Rev. Immunol 23, 683–747 (2005).
Lill, C. M. et al. CXCR5, SOX8, RPS6KB1 and ZBTB46 are genetic risk loci for multiple sclerosis. Brain 136, 1778–1782 (2013).
Ascherio, A. Environmental factors in multiple sclerosis. Expert Rev. Neurother. 13 (Suppl. 12), 3–9 (2013).
Dutta, R. & Trapp, B. D. Pathology and definition of multiple sclerosis. Rev. Prat. 56, 1293–1298 (2006).
Lassmann, H., Brück, W. & Lucchinetti, C. F. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 17, 210–218 (2007).
Dawson, J. D. The histology of disseminated sclerosis. Trans. R. Soc. Edin. 50, 517–740 (1916).
Brownell, B. & Hughes, J. T. The distribution of plaques in the cerebrum in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 25, 315–320 (1962).
Chard, D. & Miller, D. Grey matter pathology in clinically early multiple sclerosis: evidence from magnetic resonance imaging. J. Neurol. Sci. 282, 5–11 (2009).
Calabrese, M., Filippi, M. & Gallo, P. Cortical lesions in multiple sclerosis. Nature Rev. Neurol. 6, 438–444 (2010).
Kidd, D. et al. Cortical lesions in multiple sclerosis. Brain 122, 17–26 (1999).
Peterson, J. W., Bö, L., Mörk, S., Chang, A. & Trapp, B. D. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann. Neurol. 50, 389–400 (2001). The seminal paper on cortical lesions in multiple sclerosis.
Bø, L., Vedeler, C. A., Nyland, H. I., Trapp, B. D. & Mörk, S. J. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J. Neuropathol. Exp. Neurol. 62, 723–732 (2003).
Brink, B. P. et al. The pathology of multiple sclerosis is location-dependent: no significant complement activation is detected in purely cortical lesions. J. Neuropathol. Exp. Neurol. 64, 147–155 (2005).
van Horssen, J., Brink, B. P., de Vries, H. E., van der Valk, P. & Bø, L. The blood–brain barrier in cortical multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 66, 321–328 (2007).
Wegner, C., Esiri, M. M., Chance, S. A., Palace, J. & Matthews, P. M. Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology 67, 960–967 (2006).
Magliozzi, R. et al. A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann. Neurol. 68, 477 (2010). A very interesting study demonstrating a direct relationship between meningeal inflammation, subpial demyelination and neuronal loss in multiple sclerosis.
Freund, P. et al. Disability, atrophy and cortical reorganization following spinal cord injury. Brain 134, 1610–1622 (2011).
Sailer, M. et al. Focal thinning of the cerebral cortex in multiple sclerosis. Brain 126, 1734–1744 (2003).
Narayanan, S. et al. Imaging of axonal damage in multiple sclerosis: spatial distribution of magnetic resonance imaging lesions. Ann. Neurol. Mar. 41, 385–391 (1997).
Varga, A. W. et al. White matter hemodynamic abnormalities precede sub-cortical gray matter changes in multiple sclerosis. J. Neurol. Sci. 282, 28–33 (2009).
Dziedzic, T. et al. Wallerian degeneration: a major component of early axonal pathology in multiple sclerosis. Brain Pathol. 20, 976–985 (2010).
Henry, R. G. et al. Connecting white matter injury and thalamic atrophy in clinically isolated syndromes. J. Neurol. Sci. 282, 61–66 (2009).
Audoin, B. et al. Localization of grey matter atrophy in early RRMS: A longitudinal study. J. Neurol. 253, 1495–1501 (2006).
De Stefano, N. et al. Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology 60, 1157–1162 (2003). One of the seminal MRI studies on cortical atrophy in multiple sclerosis.
Furby, J. et al. Different white matter lesion characteristics correlate with distinct grey matter abnormalities on magnetic resonance imaging in secondary progressive multiple sclerosis. Mult. Scler. 15, 687–694 (2009).
Sanfilipo, M. P., Benedict, R. H., Sharma, J., Weinstock-Guttman, B. & Bakshi, R. The relationship between whole brain volume and disability in multiple sclerosis: a comparison of normalized gray versus white matter with misclassification correction. Neuroimage 26, 1068–1077 (2005).
Roosendaal, S. D. et al. Grey matter volume in a large cohort of MS patients: relation to MRI parameters and disability. Mult. Scler. 17, 1098–1106 (2011).
Ceccarelli, A. et al. A voxel-based morphometry study of grey matter loss in MS patients with different clinical phenotypes. Neuroimage 42, 315–322 (2008).
Battaglini, M. et al. Voxel-wise assessment of progression of regional brain atrophy in relapsing-remitting multiple sclerosis. J. Neurol. Sci. 282, 55–60 (2009).
Bendfeldt, K. et al. Association of regional gray matter volume loss and progression of white matter lesions in multiple sclerosis — a longitudinal voxel-based morphometry study. Neuroimage 45, 60–67 (2009).
Pagani, E. et al. Regional brain atrophy evolves differently in patients with multiple sclerosis according to clinical phenotype. Am. J. Neuroradiol. 26, 341–346 (2005).
Sepulcre, J. et al. Contribution of white matter lesions to gray matter atrophy in multiple sclerosis: evidence from voxel-based analysis of T1 lesions in the visual pathway. Arch. Neurol. 66, 173–179 (2009).
Gilmore, C. P. et al. Regional variations in the extent and pattern of grey matter demyelination in multiple sclerosis: a comparison between the cerebral cortex, cerebellar cortex, deep grey matter nuclei and the spinal cord. J. Neurol. Neurosurg. Psychiatry 80, 182–187 (2009).
Kutzelnigg, A. et al. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol. 17, 38–44 (2007).
Gilmore, C. P. et al. Spinal cord neuronal pathology in multiple sclerosis. Brain Pathol. 19, 642–649 (2009).
Howell, O. et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 134, 2755–2771 (2011).
Lucchinetti, C. F. et al. Inflammatory cortical demyelination in early multiple sclerosis. N. Engl. J. Med. 365, 2188–2197 (2011). The first neuropathological characterization of inflammatory cortical lesions in early multiple sclerosis.
Calabrese, M. et al. Detection of cortical inflammatory lesions by double inversion recovery magnetic resonance imaging in patients with multiple sclerosis. Arch. Neurol. 64, 1416–1422 (2007).
Giorgio, A. et al. Cortical lesions in radiologically isolated syndrome. Neurology 77, 1896–1899 (2011).
Calabrese, M. & Gallo, P. Magnetic resonance evidence of cortical onset of multiple sclerosis. Mult. Scler. 15, 933–941 (2009).
Seewann, A. et al. Imaging the tip of the iceberg: visualization of cortical lesions in multiple sclerosis. Mult. Scler. 17, 1202–1210 (2011).
Chard, D. T. et al. Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain 125, 327–337 (2002).
Tiberio, M. et al. Gray and white matter volume changes in early RRMS: a 2-year longitudinal study. Neurology 64, 1001–1007 (2005).
Sbardella, E. et al. Assessing the correlation between grey and white matter damage with motor and cognitive impairment in multiple sclerosis patients. PLoS ONE 8, e63250 (2013).
Steenwijk, M. D. et al. What explains gray matter atrophy in long-standing multiple sclerosis? Radiology 272, 832–842 (2014).
Calabrese, M. et al. Imaging distribution and frequency of CLs in patients with multiple sclerosis. Neurology 75, 1234–1240 (2010).
Vercellino, M. et al. Demyelination, inflammation, and neurodegeneration in multiple sclerosis deep gray matter. J. Neuropathol. Exp. Neurol. 68, 489–502 (2009).
Geurts, J. J. et al. Extensive hippocampal demyelination in multiple sclerosis. J. Neuropathol. Exp. Neurol. 66, 819–827 (2007).
Cohen-Adad, J. et al. In vivo evidence of disseminated subpial T2* signal changes in multiple sclerosis at 7 T: a surface-based analysis. Neuroimage 57, 55–62 (2011).
Audoin, B. et al. Atrophy mainly affects the limbic system and the deep grey matter at the first stage of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 81, 690–695 (2010).
Bendfeldt, K. et al. Spatiotemporal distribution pattern of white matter lesion volumes and their association with regional grey matter volume reductions in relapsing-remitting multiple sclerosis. Hum. Brain Mapp. 31, 1542–1555 (2010).
Bettelli, E. et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197, 1073–1081 (2003).
Krishnamoorthy, G. et al. Myelin-specific T cells also recognize neuronal autoantigen in a transgenic mouse model of multiple sclerosis. Nature Med. 15, 626–632 (2009).
Serafini, B., Rosicarelli, B., Magliozzi, R., Stigliano, E. & Aloisi, F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 14, 164–174 (2004).
Ascherio, A. & Munger, K. L. Epstein–Barr virus infection and multiple sclerosis: a review. J. Neuroimmune Pharmacol. 5, 271–277 (2010).
Ascherio, A. et al. Epstein–Barr virus antibodies and risk of multiple sclerosis: a prospective study. JAMA 286, 3083–3088 (2001).
Levin, L. I. et al. Multiple sclerosis and Epstein–Barr virus. JAMA 289, 1533–1536 (2003).
Serafini, B. et al. Dysregulated Epstein–Barr virus infection in the multiple sclerosis brain. J. Exp. Med. 204, 2899–2912 (2007).
Serafini, B., Muzio, L., Rosicarelli, B. & Aloisi, F. Radioactive in situ hybridization for Epstein–Barr virus-encoded small RNA supports presence of Epstein–Barr virus in the multiple sclerosis brain. Brain 136, e233 (2013).
Magliozzi, R. et al. B-cell enrichment and Epstein–Barr virus infection in inflammatory cortical lesions in secondary progressive multiple sclerosis. J. Neuropathol. Exp. Neurol. 72, 29–41 (2013).
Angelini, D. F. et al. Increased CD8+ T cell response to Epstein–Barr virus lytic antigens in the active phase of multiple sclerosis. PLoS Pathog. 9, e1003220 (2013).
Lossius, A. et al. High-throughput sequencing of TCR repertoires in multiple sclerosis reveals intrathecal enrichment of EBV-reactive CD8+ T cells. Eur. J. Immunol. 44, 3439–3452 (2014).
Sargsyan, S. A. et al. Absence of Epstein–Barr virus in the brain and CSF of patients with multiple sclerosis. Neurology 74, 1127–1135 (2010).
Willis, S. N. et al. Epstein–Barr virus infection is not a characteristic feature of multiple sclerosis brain. Brain 132, 3318–3328 (2009).
Lassmann, H., Niedobitek, G., Aloisi, F., Middeldorp, J. M. & NeuroproMiSe EBV Working Group. Epstein–Barr virus in the multiple sclerosis brain: a controversial issue — report on a focused workshop held in the Centre for Brain Research of the Medical University of Vienna, Austria. Brain 134, 2772–2786 (2011).
Aloisi, F., Serafini, B., Magliozzi, R., Howell, O. W. & Reynolds, R. Detection of Epstein–Barr virus and B-cell follicles in the multiple sclerosis brain: what you find depends on how and where you look. Brain 133, e157 (2010).
Maggi, F. et al. Low prevalence of TT virus in the cerebrospinal fluid of viremic patients with central nervous system disorders. J. Med. Virol. 65, 418–422 (2001).
Sospedra, M. et al. Recognition of conserved amino acid motifs of common viruses and its role in autoimmunity. PLoS Pathog. 1, e41 (2005).
Lamberto, I., Gunst, K., Müller, H., Zur Hausen, H. & de Villiers, E. M. Mycovirus-like DNA virus sequences from cattle serum and human brain and serum samples from multiple sclerosis patients. Genome Announc. 2, e00848–14 (2014).
Borkosky, S. S., Whitley, C., Kopp-Schneider, A., zur Hausen, H. & de Villiers, E. M. Epstein–Barr virus stimulates torque teno virus replication: a possible relationship to multiple sclerosis. PLoS ONE 7, e32160 (2012).
Zeis, T., Graumann, U., Reynolds, R. & Schaeren-Wiemers, N. Normal-appearing white matter in multiple sclerosis is in a subtle balance between inflammation and neuroprotection. Brain 131, 288–303 (2008).
Baranzini, S. E. et al. Genetic variation influences glutamate concentrations in brains of patients with multiple sclerosis. Brain 133, 2603–2611 (2010).
Mastronardi, F. G. & Moscarello, M. A. Molecules affecting myelin stability: a novel hypothesis regarding the pathogenesis of multiple sclerosis. J. Neurosci. Res. 80, 301–308 (2005).
Neumann, H., Cavalié, A., Jenne, D. E. & Wekerle, H. Induction of MHC class I genes in neurons. Science 269, 549–552 (1995).
Jersild, C. et al. Histocompatibility determinants in multiple sclerosis, with special reference to clinical course. Lancet 2, 1221–1225 (1973).
The International Multiple Sclerosis Genetics Consortium (IMSGC) et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476, 214–219 (2011).
Martin, R. et al. Fine specificity and HLA restriction of myelin basic protein-specific cytotoxic T cell lines from multiple sclerosis patients and healthy individuals. J. Immunol. 145, 540–548 (1990).
Ota, K. et al. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature 346, 183–187 (1990).
Bielekova, B. et al. Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J. Immunol. 172, 3893–3904 (2004).
Yates, R. L., Esiri, M. M., Palace, J., Mittal, A. & DeLuca, G. C. The influence of HLA-DRB1*15 on motor cortical pathology in multiple sclerosis. Neuropathol. Appl. Neurobiol. http://dx.doi.org/10.1111/nan.12165 (2014).
Höftberger, R. et al. Expression of major histocompatibility complex class I molecules on the different cell types in multiple sclerosis lesions. Brain Pathol. 14, 43–50 (2004).
Babbe, H. et al. Clonal expansions of CD8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J. Exp. Med. 192, 393–404 (2000).
Ulvestad, E. et al. HLA class II molecules (HLA-DR, -DP, -DQ) on cells in the human CNS studied in situ and in vitro. Immunology 82, 535–541 (1994).
Liblau, R. S., Gonzalez-Dunia, D., Wiendl, H. & Zipp, F. Neurons as targets for T cells in the nervous system. Trends Neurosci. 36, 315–324 (2013).
Meuth, S. G. et al. Cytotoxic CD8+ T cell-neuron interactions: perforin-dependent electrical silencing precedes but is not causally linked to neuronal cell death. J. Neurosci. 29, 15397–15409 (2009).
Suidan, H. S. et al. Granzyme A released upon stimulation of cytotoxic T lymphocytes activates the thrombin receptor on neuronal cells and astrocytes. Proc. Natl Acad. Sci. USA 91, 8112–8116 (1994).
Medana, I. M. et al. MHC class I-restricted killing of neurons by virus-specific CD8+ T lymphocytes is effected through the Fas/FasL, but not the perforin pathway. Eur. J. Immunol. 30, 3623–3633 (2000).
Aktas, O. et al. Neuronal damage in autoimmune neuroinflammation mediated by the death ligand TRAIL. Neuron 46, 421–432 (2005).
Mizuno, T. et al. Interferon-gamma directly induces neurotoxicity through a neuron specific, calcium-permeable complex of IFN-γ receptor and AMPA GluR1 receptor. FASEB J. 22, 1797–1806 (2008).
Vergelli, M. et al. Human autoreactive CD4+ T cell clones use perforin- or Fas/Fas ligand-mediated pathways for target cell lysis. J. Immunol. 158, 2756–2761 (1997).
Vergelli, M. et al. A novel population of CD4+CD56+ myelin-reactive T cells lyses target cells expressing CD56/neural cell adhesion molecule. J. Immunol. 157, 679–688 (1996).
Zaguia, F. et al. Cytotoxic NKG2C+ CD4 T cells target oligodendrocytes in multiple sclerosis. J. Immunol. 190, 2510–2518 (2013).
Magliozzi, R. et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130, 1089–1104 (2007).
Magliozzi, R., Columba-Cabezas, S., Serafini, B. & Aloisi, F. Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J. Neuroimmunol. 148, 11–23 (2004).
Peters, A. et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity 35, 986–996 (2011).
Choi, S. R. et al. Meningeal inflammation plays a role in the pathology of primary progressive multiple sclerosis. Brain 135, 2925–2937 (2012).
Kramann, N. et al. Increased meningeal T and plasma cell infiltration is associated with early subpial cortical demyelination in common marmosets with experimental autoimmune encephalomyelitis. Brain Pathol. http://dx.doi.org/10.1111/bpa.12180 (2014).
Gardner, C. et al. Cortical grey matter demyelination can be induced by elevated pro-inflammatory cytokines in the subarachnoid space of MOG-immunized rats. Brain 136, 3596–3608 (2013).
Kooi, E. J., Geurts, J. J., van Horssen, J., Bø, L. & van der Valk, P. Meningeal inflammation is not associated with cortical demyelination in chronic multiple sclerosis. J. Neuropathol. Exp. Neurol. 68, 1021–1028 (2009).
Guseo, A. & Jellinger, K. The significance of perivascular infiltrations in multiple sclerosis. J. Neurol. 211, 51–60 (1975).
Kutzelnigg, A. et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128, 2705–2712 (2005). This study provides elegant autoptic evidence of white matter and grey matter damage in multiple sclerosis.
Reynolds, R. et al. The neuropathological basis of clinical progression in multiple sclerosis. Acta Neuropathol. 122, 155–170 (2011).
Fischer, M. T. et al. Disease-specific molecular events in cortical multiple sclerosis lesions. Brain 136, 1799–1815 (2013).
Calabrese, M. et al. The changing clinical course of multiple sclerosis: a matter of gray matter. Ann. Neurol. 74, 76–83 (2013). An interesting paper implicating grey matter damage in the progression of clinical disability during the course of multiple sclerosis.
Androdias, G. et al. Meningeal T cells associate with diffuse axonal loss in multiple sclerosis spinal cords. Ann. Neurol. 68, 465–476 (2010).
Dutta, R. & Trapp, B. D. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology 68 (Suppl. 3), 22–31 (2007).
Kreutzberg, G. W. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 19, 312–318 (1996).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
Aloisi, F. Immune function of microglia. Glia 36, 1 65–179 (2001).
Block, M. L. & Hong, J. S. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem. Soc. Trans. 35, 1127–1132 (2007).
Polazzi, E. & Contestabile, A. Reciprocal interactions between microglia and neurons: from survival to neuropathology. Rev. Neurosci. 13, 221–242 (2002).
Gray, E., Thomas, T. L., Betmouni, S., Scolding, N. & Love, S. Elevated matrix metalloproteinase-9 and degradation of perineuronal nets in cerebrocortical multiple sclerosis plaques. J. Neuropathol. Exp. Neurol. 67, 888–899 (2008).
Vercellino, M. et al. Altered glutamate reuptake in relapsing-remitting and secondary progressive multiple sclerosis cortex: correlation with microglia infiltration, demyelination, and neuronal and synaptic damage. J. Neuropathol. Exp. Neurol. 66, 732–739 (2007).
Kooi, E. J., Strijbis, E. M., van der Valk, P. & Geurts, J. J. Heterogeneity of cortical lesions in multiple sclerosis: clinical and pathologic implications. Neurology 79, 1369–1376 (2012).
Stys, P. K., Zamponi, G. W., van Minnen, J. & Geurts, J. J. Will the real multiple sclerosis please stand up? Nature Rev. Neurosci. 13, 507–514 (2012).
Barnett, M. H. & Prineas, J. W. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann. Neurol. 55, 458–468 (2004).
Henderson, A. P., Barnett, M. H., Parratt, J. D. & Prineas, J. W. Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann. Neurol. 66, 739–753 (2009).
Lassmann, H., van Horssen, J. & Mahad, D. Progressive multiple sclerosis: pathology and pathogenesis. Nature Rev. Neurol. 8, 647–656 (2012). A comprehensive review on the pathology and pathogenesis of progressive multiple sclerosis.
Fischer, M. T. et al. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 135, 886–899 (2012).
Witte, M. E., Geurts, J. J., de Vries, H. E., van der Valk, P. & van Horssen, J. Mitochondrial dysfunction: a potential link between neuroinflammation and neurodegeneration? Mitochondrion 10, 411–418 (2010).
Campbell, G. R. et al. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann. Neurol. 69, 481–492 (2011).
Nikic, I. et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nature Med. 17, 495–499 (2011).
van Horssen, J., Witte, M. E. & Ciccarelli, O. The role of mitochondria in axonal degeneration and tissue repair in MS. Mult. Scler. 18, 1058–1067 (2012).
Mahad, D., Ziabreva, I., Lassmann, H. & Turnbull, D. Mitochondrial defects in acute multiple sclerosis lesions. Brain 131, 1722–1735 (2008).
Marik, C., Felts, P. A., Bauer, J., Lassmann, H. & Smith, K. J. Lesion genesis in a subset of patients with multiple sclerosis: a role for innate immunity? Brain 130, 2800–2815 (2007).
Druzhyna, N. M., Wilson, G. L. & LeDoux, S. P. Mitochondrial DNA repair in aging and disease. Mech. Ageing Dev. 129, 383–390 (2008).
Trapp, B. D. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 8, 280–291 (2009).
Acknowledgements
M.C. is supported by the Progressive MS Alliance (PA-0124). R.Magliozzi is supported by an Italian Multiple Sclerosis Foundation grant (FISM 2011/R/23) and by an Italian Ministry of Health grant (GR-2010-2313255). R.R. is supported by the UK Multiple Sclerosis Society and the UK Medical Research Council. R. Martin and the Neuroimmunology and Multiple Sclerosis Research Section are supported by the Clinical Research Priority Program MS (CRPPMS) of the University of Zurich, the Swiss National Science Foundation (SNF), a European Research Council (ERC) Advanced Grant, the EU-FP7 framework programme and the Swiss Multiple Sclerosis Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M.C. has received speaking and consultancy honoraria from Biogen Idec, Merck Serono, Genzyme (a Sanofi company), and Teva Pharmaceutical companies. J.J.G.G. has received research support from Novartis and Biogen Idec and was a consultant for Novartis, Biogen Idec, Genzyme, Merck Serono, and Teva Pharmaceutical companies.
Glossary
- Complement proteins
-
A set of plasma proteins that coats pathogens; the coated pathogens are then cleared by phagocytes.
- MRI
-
(Magnetic resonance imaging). A non-invasive method used to obtain images of living tissue. It uses radio-frequency pulses and magnetic field gradients; the principle of nuclear magnetic resonance is used to reconstruct images of tissue characteristics (for example, proton density or water diffusion parameters).
- Wallerian degeneration
-
The degeneration of an axon distal to a site of injury, which begins to occur approximately 1.5 days after the injury.
- Clinically isolated syndrome
-
A syndrome present in a patient experiencing their first clinical episode that is suggestive of an inflammatory demyelinating disease of the CNS.
- Relapsing–remitting multiple sclerosis
-
(RRMS). The early phase of multiple sclerosis characterized by several neurological episodes followed by complete or incomplete recovery.
- Radiologically isolated syndrome
-
A syndrome present in a patient who has radiological evidence of an inflammatory demyelinating disease of the CNS but no clinical signs or symptoms of such disease.
- Primary and secondary progressive disease
-
Phases of multiple sclerosis characterized by a slow progression of disability without a well-defined clinical relapse. These phases usually follow the relapsing–remitting phase (secondary progressive phase) but they can also be in the first phase of the disease (primary progressive multiple sclerosis).
- T cell
-
A lymphocyte that mediates cell-dependent immune responses by providing help (in the form of cytokines, for example) to other immune cells or by cytotoxicity (killing of a virus-infected cell).
- B cells
-
Lymphocytes that express immunoglobulins as surface receptors or, when they are fully mature following antigenic stimulation, release antibodies that are directed against a virus or bacteria.
- Natural killer cells
-
A white blood cell population that does not express antigen-specific recognition receptors such as those expressed by T and B cells, but recognizes cells that express fewer or no HLA class I molecules (such as virus-infected cells). Natural killer cells are important in controlling viral infections and recognition of mutated (tumour) cells.
- Experimental autoimmune encephalomyelitis
-
An animal model of multiple sclerosis that is initiated in animals by injecting myelin proteins or peptides to raise autoreactive T cells or by the transfer of autoreactive T cells into naive recipients.
Rights and permissions
About this article
Cite this article
Calabrese, M., Magliozzi, R., Ciccarelli, O. et al. Exploring the origins of grey matter damage in multiple sclerosis. Nat Rev Neurosci 16, 147–158 (2015). https://doi.org/10.1038/nrn3900
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3900
This article is cited by
-
Magnetic Resonance Imaging Evidence Supporting the Efficacy of Cladribine Tablets in the Treatment of Relapsing-Remitting Multiple Sclerosis
CNS Drugs (2024)
-
Imaging of brain barrier inflammation and brain fluid drainage in human neurological diseases
Cellular and Molecular Life Sciences (2024)
-
Meningeal inflammation as a driver of cortical grey matter pathology and clinical progression in multiple sclerosis
Nature Reviews Neurology (2023)
-
CNS endothelial derived extracellular vesicles are biomarkers of active disease in multiple sclerosis
Fluids and Barriers of the CNS (2022)
-
Mechanism-based criteria to improve therapeutic outcomes in progressive multiple sclerosis
Nature Reviews Neurology (2022)