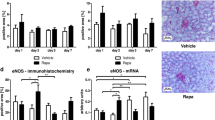
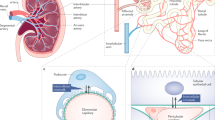
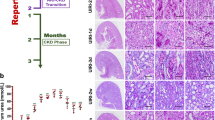
Abstract
Chronic kidney disease is characterized by progressive loss of the renal microvasculature, which leads to local areas of hypoxia and induction of profibrotic responses, scarring and deterioration of renal function. Revascularization alone might be sufficient to restore kidney function and regenerate the structure of the diseased kidney. For revascularization to be successful, however, the underlying disease process needs to be halted or alleviated and there must remain a sufficient number of surviving nephron units that can serve as a scaffold for kidney regeneration. This Perspectives article describes how revascularization might be achieved using vascular growth factors or adoptive transfer of endothelial progenitor cells and provides a brief outline of the studies performed to date. An overview of how therapeutic strategies targeting the microvasculature could be enhanced in the future is also presented.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Coresh, J., Astor, B. C., Greene, T., Eknoyan, G. & Levey, A. S. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am. J. Kidney Dis. 41, 1–12 (2003).
Fine, L. G., Orphanides, C. & Norman, J. T. Progressive renal disease: the chronic hypoxia hypothesis. Kidney Int. Suppl. 65, S74–S78 (1998).
Nangaku, M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J. Am. Soc. Nephrol. 17, 17–25 (2006).
Carmeliet, P. & Jain, R. K. Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 (2011).
Maeshima, Y. & Makino, H. Angiogenesis and chronic kidney disease. Fibrogenesis Tissue Repair 3, 13 (2010).
Mayer, G. Capillary rarefaction, hypoxia, VEGF and angiogenesis in chronic renal disease. Nephrol. Dial. Transplant. 26, 1132–1137 (2011).
Kang, D. H., Hughes, J., Mazzali, M., Schreiner, G. F. & Johnson, R. J. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J. Am. Soc. Nephrol. 12, 1448–1457 (2001).
Iliescu, R., Fernandez, S. R., Kelsen, S., Maric, C. & Chade, A. R. Role of renal microcirculation in experimental renovascular disease. Nephrol. Dial. Transplant. 25, 1079–1087 (2010).
Kim, W. et al. COMP-Angiopoietin-1 ameliorates renal fibrosis in a unilateral ureteral obstruction model. J. Am. Soc. Nephrol. 17, 2474–2483 (2006).
Long, D. A. et al. Angiopoietin-1 therapy enhances fibrosis and inflammation following folic acid-induced acute renal injury. Kidney Int. 74, 300–309 (2008).
Sato, W. et al. Selective stimulation of VEGFR2 accelerates progressive renal disease. Am. J. Pathol. 179, 155–166 (2011).
Schrijvers, B. F. et al. Pathophysiological role of vascular endothelial growth factor in the remnant kidney. Nephron Exp. Nephrol. 101, e9–e15 (2005).
Nakagawa, T., Kosugi, T., Haneda, M., Rivard, C. J. & Long, D. A. Abnormal angiogenesis in diabetic nephropathy. Diabetes 58, 1471–1478 (2009).
Saito, D. et al. Amelioration of renal alterations in obese type 2 diabetic mice by vasohibin-1, a negative feedback regulator of angiogenesis. Am. J. Physiol. Renal Physiol. 300, F873–F886 (2011).
Leonard, E. C., Friedrich, J. L. & Basile, D. P. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am. J. Physiol. Renal Physiol. 295, F1648–F1657 (2008).
Mu, W. et al. Angiostatin overexpression is associated with an improvement in chronic kidney injury by an anti-inflammatory mechanism. Am. J. Physiol. Renal Physiol. 296, F145–F152 (2009).
Bottinger, E. P. TGF-beta in renal injury and disease. Semin. Nephrol. 27, 309–320 (2007).
Kawai, H. et al. Lack of the growth factor midkine enhances survival against cisplatin-induced renal damage. Am. J. Pathol. 165, 1603–1612 (2004).
Denby, L. et al. Development of renal-targeted vector through combined in vivo phage display and capsid engineering of adenoviral fibers from serotype 19p. Mol. Ther. 15, 1647–1654 (2007).
Korpisalo, P. et al. Capillary enlargement, not sprouting angiogenesis, determines beneficial therapeutic effects and side effects of angiogenic gene therapy. Eur. Heart J. 32, 1664–1672 (2011).
Nagy, J. A. et al. Permeability properties of tumor surrogate blood vessels induced by VEGF.-A. Lab. Invest. 86, 767–780 (2006).
Gavard, J., Patel, V. & Gutkind, J. S. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev. Cell 14, 25–36 (2008).
Elson, D. A. et al. Induction of hypervascularity without leakage or inflammation in transgenic mice overexpressing hypoxia-inducible factor-1. Genes Dev. 15, 2520–2532 (2001).
Tanaka, T. et al. Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab. Invest. 85, 1292–1307 (2005).
Higgins, D. F. et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Invest. 117, 3810–3820 (2007).
Bates, D. O. et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 62, 4123–4131 (2002).
Manetti, M. et al. Overexpression of VEGF165b, an inhibitory splice variant of vascular endothelial growth factor leads to insufficient angiogenesis in patients with systemic sclerosis. Circ. Res. 109, e14–e26 (2011).
Ghosh, G. et al. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-α isoforms and promotes angiogenesis. J. Clin. Invest. 120, 4141–4154 (2010).
Anand, S. et al. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angigogenesis. Nat. Med. 16, 909–914 (2010).
Dwivedi, R. S., Herman, J. G., McCaffrey, T. A. & Raj, D. S. Beyond genetics: epigenetic code in chronic kidney disease. Kidney Int. 79, 23–32 (2011).
Jakobsson, L. et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell. Biol. 12, 943–953 (2010).
Lin, S. L. et al. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am. J. Pathol. 178, 911–923 (2011).
Armulik, A., Abramsson, A. & Betsholtz C. Endothelial/pericyte interactions. Circ. Res. 97, 512–523 (2005).
Chen, J. et al. Kidney-derived mesenchymal stem cells contribute to vasculogenesis, angiogenesis and endothelial repair. Kidney Int. 74, 879–889 (2008).
Bussolati, B. et al. Isolation of renal progenitor cells from adult human kidney. Am. J. Pathol. 166, 545–555 (2005).
Yasuda, K. et al. Adriamycin nephropathy, a failure of endothelial progenitor cell-induced repair. Am. J. Pathol. 176, 1685–1695 (2010).
Oliver, J. Architecture of the kidney in chronic Bright's disease (Paul B. Hoeber Inc., New York, 1939).
Fine, L. G. First heal thyself: Rescue of dysfunctional endothelial progenitor cells restores function to the injured kidney. Am. J. Pathol. 176, 1586–1587 (2010).
Asahara, T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 (1997).
Grant, M. B. et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat. Med. 8, 607–612 (2002).
Hirschi, K. K., Ingram, D. A. & Yoder, M. C. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 28, 1584–1595 (2008).
Goligorsky, M. S., Yasuda, K. & Ratliff, B. Dysfunctional endothelial progenitor cells in chronic kidney disease. J. Am. Soc. Nephrol. 21, 911–919 (2010).
Togel, F., Issac, J., Hu, Z., Weiss, K. & Westernfelder, C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 67, 1772–1784 (2005).
Chade, A. R. et al. Endothelial progenitor cells homing and renal repair in experimental renovascular disease. Stem Cells 28, 1039–1047 (2010).
Zhu, X. Y. et al. Enhanced endothelial progenitor cell angiogenic potency, present in early experimental renovascular hypertension, deteriorates with disease duration. J. Hypertens. 29, 1972–1979 (2011).
Krenning, G. et al. Endothelial progenitor cell dysfunction in patients with progressive chronic kidney disease. Am. J. Physiol. Renal Physiol. 296, F1314–F1322 (2009).
Sangidorj, O. et al. Bone marrow-derived endothelial progenitor cells confer renal protection in a murine chronic renal failure model. Am. J. Physiol. Renal Physiol. 299, F325–F335 (2010).
Chade, A. R. et al. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation 119, 547–557 (2009).
Medici, D. et al. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat. Med. 16, 1400–1406 (2010).
Yoon, C.-H. et al. Mechanism of improved cardiac function after bone marrow mononuclear cell therapy: role of cardiovascular lineage commitment. Circulation 121, 2001–2011 (2010).
Sakamoto, I. et al. Lymphatic vessels develop during tubulointerstitial fibrosis. Kidney Int. 75, 828–838 (2009).
Matsui, K. et al. Lymphatic microvessels in the rat remnant kidney model of renal fibrosis: aminopeptidase p and podoplanin are discriminatory markers for endothelial cells of blood and lymphatic vessels. J. Am. Soc. Nephrol. 14, 1981–1989 (2003).
Acknowledgements
This work was supported by a Kidney Research UK Senior Non-Clinical Fellowship and a Medical Research Council New Investigator Award to D. A. Long and research support for L. G. Fine from Cedars-Sinai Medical Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Long, D., Norman, J. & Fine, L. Restoring the renal microvasculature to treat chronic kidney disease. Nat Rev Nephrol 8, 244–250 (2012). https://doi.org/10.1038/nrneph.2011.219
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2011.219
This article is cited by
-
Hypoxic preconditioning induces epigenetic changes and modifies swine mesenchymal stem cell angiogenesis and senescence in experimental atherosclerotic renal artery stenosis
Stem Cell Research & Therapy (2021)
-
Human amniotic epithelial cells ameliorate kidney damage in ischemia-reperfusion mouse model of acute kidney injury
Stem Cell Research & Therapy (2020)
-
Protecting the kidney in systemic lupus erythematosus: from diagnosis to therapy
Nature Reviews Rheumatology (2020)
-
Magnesium lithospermate B improves renal hemodynamics and reduces renal oxygen consumption in 5/6th renal ablation/infarction rats
BMC Nephrology (2019)
-
Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects
GeroScience (2019)