Abstract
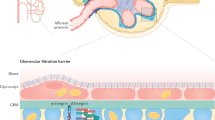
The architectural design of our kidneys is amazingly complex, and culminates in the 3D structure of the glomerular filter. During filtration, plasma passes through a sieve consisting of a fenestrated endothelium and a broad basement membrane before it reaches the most unique part, the slit diaphragm, a specialized type of intercellular junction that connects neighbouring podocyte foot processes. When podocytes become stressed, irrespective of the causative stimulus, they undergo foot process effacement and loss of slit diaphragms—two key steps leading to proteinuria. Thus, proteinuria is the unifying denominator of a broad spectrum of podocytopathies. With the rising prevalence of chronic kidney disease and the fact that glomerular diseases account for the majority of patients with end-stage renal disease, further investigation and elucidation of this unique structure is of paramount importance. This Review recounts how perception of the slit diaphragm has changed over time as a result of intense research, from its first anatomical description as a thin intercellular connection, to an appreciation of its role as a dynamic signalling hub. These observations led to the introduction of novel concepts in podocyte biology, which could pave the way to development of highly desired, specific therapeutic strategies for glomerular diseases.
Key Points
-
The slit diaphragm is a unique intercellular contact point that integrates the structural components of various other cell junction types, including tight, adhesion, gap and neuronal junctions
-
We propose that the slit diaphragm should be classified as a unique category of intercellular junction
-
Slit diaphragm functioning is impaired in all monogenic inherited proteinuric diseases
-
The slit diaphragm executes several highly specialized functions: macromolecular filtering, connecting the slit diaphragm to the actin cytoskeleton and initiating signalling pathways that regulate the plasticity of foot processes
-
The slit diaphragm also mediates calcium signalling, mechanosensation, regulation of the cytoskeleton, cell polarity, cell survival, endocytosis and transcription
-
Further research into this delicate structure holds the potential to discover novel treatment options for proteinuria and chronic kidney diseases
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
25 November 2012
In the original 'about the authors section', author affiliations for Christoph Schell and Tobias Huber were not complete. This has been corrected for the online version of the article.
References
Pavenstädt, H., Kriz, W. & Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 83, 253–307 (2003).
Potter, E. L. Development of the human glomerulus. Arch. Pathol. 80, 241–255 (1965).
Pak Poy, R. K. & Robertson, J. S. Electron microscopy of the avian renal glomerulus. J. Biophys. Biochem. Cytol. 3, 183–192 (1957).
Pak Poy, R. K. Electron microscopy of the marsupial renal glomerulus. Aust. J. Exp. Biol. Med. Sci. 35, 437–447 (1957).
Farquhar, M. G. & Palade, G. E. Glomerular permeability. II. Ferritin transfer across the glomerular capillary wall in nephrotic rats. J. Exp. Med. 114, 699–716 (1961).
Vernier, R. L., Papermaster, B. W. & Good, R. A. Aminonucleoside nephrosis. I. Electron microscopic study of the renal lesion in rats. J. Exp. Med. 109, 115–126 (1959).
Caulfield, J. P. & Farquhar, M. G. The permeability of glomerular capillaries to graded dextrans. Identification of the basement membrane as the primary filtration barrier. J. Cell Biol. 63, 883–903 (1974).
Farquhar, M. G., Wissig, S. L. & Palade, G. E. Glomerular permeability. I. Ferritin transfer across the normal glomerular capillary wall. J. Exp. Med. 113, 47–66 (1961).
Venkatachalam, M. A., Cotran, R. S. & Karnovsky, M. J. An ultrastructural study of glomerular permeability in aminonucleoside nephrosis using catalase as a tracer protein. J. Exp. Med. 132, 1168–1180 (1970).
Yamada, E. The fine structure of the renal glomerulus of the mouse. J. Biophys. Biochem. Cytol. 1, 551–566 (1955).
Venkatachalam, M. A., Karnovsky, M. J., Fahimi, H. D. & Cotran, R. S. An ultrastructural study of glomerular permeability using catalase and peroxidase as tracer proteins. J. Exp. Med. 132, 1153–1167 (1970).
Hall, B. V. A slit pore theory of capillary filtration based on electron micrographic data on the filtration pathway through the cellular layers of mammalian glomerular capillary walls. Trans. Am. Microsc. Soc. 96, 413–438 (1977).
Rodewald, R. & Karnovsky, M. J. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J. Cell Biol. 60, 423–433 (1974).
Karnovsky, M. J. & Ryan, G. B. Substructure of the glomerular slit diaphragm in freeze-fractured normal rat kidney. J. Cell Biol. 65, 233–236 (1975).
Robson, A. M., Giangiacomo, J., Kienstra, R. A., Naqvi, S. T. & Ingelfinger, J. R. Normal glomerular permeability and its modification by minimal change nephrotic syndrome. J. Clin. Invest. 54, 1190–1199 (1974).
Caulfield, J. P., Reid, J. J. & Farquhar, M. G. Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab. Invest. 34, 43–59 (1976).
Wiggins, R. C. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 71, 1205–1214 (2007).
Kestila, M. et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol. Cell 1, 575–582 (1998).
Wartiovaara, J. et al. Nephrin strands contribute to a porous slit diaphragm scaffold as revealed by electron tomography. J. Clin. Invest. 114, 1475–1483 (2004).
Gagliardini, E., Conti, S., Benigni, A., Remuzzi, G. & Remuzzi, A. Imaging of the porous ultrastructure of the glomerular epithelial filtration slit. J. Am. Soc. Nephrol. 21, 2081–2089 (2010).
Ruotsalainen, V. et al. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc. Natl Acad. Sci. USA 96, 7962–7967 (1999).
Boute, N. et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 24, 349–354 (2000).
Donoviel, D. B. et al. Proteinuria and perinatal lethality in mice lacking Neph1, a novel protein with homology to nephrin. Mol. Cell Biol. 21, 4829–4836 (2001).
Ciani, L., Patel, A., Allen, N. D. & ffrench-Constant, C. Mice lacking the giant protocadherin mFAT1 exhibit renal slit junction abnormalities and a partially penetrant cyclopia and anophthalmia phenotype. Mol. Cell Biol. 23, 3575–3582 (2003).
Reiser, J. et al. TrpC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 37, 739–744 (2005).
Winn, M. P. et al. A mutation in the TrpC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801–1804 (2005).
Shih, N. Y. et al. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286, 312–315 (1999).
Hinkes, B. et al. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat. Genet. 38, 1397–1405 (2006).
Genovese, G. et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329, 841–845 (2010).
Kaplan, J. M. et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat. Genet. 24, 251–256 (2000).
Ozaltin, F. et al. Disruption of PTPRO causes childhood-onset nephrotic syndrome. Am. J. Hum. Genet. 89, 139–147 (2011).
Diomedi-Camassei, F. et al. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J. Am. Soc. Nephrol. 18, 2773–2780 (2007).
Heeringa, S. F. et al. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J. Clin. Invest. 121, 2013–2024 (2011).
Brown, E. J. et al. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat. Genet. 42, 72–76 (2010).
Mele, C. et al. MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N. Engl. J. Med. 365, 295–306.
Akilesh, S. et al. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J. Clin. Invest. 121, 4127–4137 (2011).
Gupta, I. R. et al. ARHGDIA: a novel gene implicated in nephrotic syndrome. J. Med. Genet. 50, 330–338 (2013).
Pelletier, J. et al. Germline mutations in the Wilms' tumour suppressor gene are associated with abnormal urogenital development in Denys–Drash syndrome. Cell 67, 437–447 (1991).
Zenker, M. et al. Human laminin β2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum. Mol. Genet. 13, 2625–2632 (2004).
Vidal, F. et al. Integrin β 4 mutations associated with junctional epidermolysis bullosa with pyloric atresia. Nat. Genet. 10, 229–234 (1995).
Berkovic, S. F. et al. Array-based gene discovery with three unrelated subjects shows SCARB2/LIMP-2 deficiency causes myoclonus epilepsy and glomerulosclerosis. Am. J. Hum. Genet. 82, 673–684 (2008).
Lopez, L. C. et al. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am. J. Hum. Genet. 79, 1125–1129 (2006).
Goto, Y., Nonaka, I. & Horai, S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 348, 651–653 (1990).
Pavlakis, S. G., Phillips, P. C., DiMauro, S., DeVivo, D. C. & Rowland, L. P. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann. Neurol. 16, 481–488 (1984).
Chen, H. et al. Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat. Genet. 19, 51–55 (1998).
Dreyer, S. D. et al. Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nat. Genet. 19, 47–50 (1998).
Boerkoel, C. F. et al. Mutant chromatin remodeling protein SMARCAL1 causes Schimke immuno-osseous dysplasia. Nat. Genet. 30, 215–220 (2002).
Bick, D. & Dimmock, D. Whole exome and whole genome sequencing. Curr. Opin. Paediatr. 23, 594–600 (2011).
Mangos, S. & Reiser, J. Fishing for new glomerular disease-related genes. J. Am. Soc. Nephrol. 22, 1960–1962 (2011).
Greka, A. & Mundel, P. Cell biology and pathology of podocytes. Annu. Rev. Physiol. 74, 299–323 (2012).
Moeller, M. J., Sanden, S. K., Soofi, A., Wiggins, R. C. & Holzman, L. B. Podocyte-specific expression of Cre recombinase in transgenic mice. Genesis 35, 39–42 (2003).
Eremina, V., Wong, M. A., Cui, S., Schwartz, L. & Quaggin, S. E. Glomerular-specific gene excision in vivo. J. Am. Soc. Nephrol. 13, 788–793 (2002).
Huber, T. B. & Benzing, T. The slit diaphragm: a signalling platform to regulate podocyte function. Curr. Opin. Nephrol. Hypertens. 14, 211–216 (2005).
Yaddanapudi, S. et al. CD2AP in mouse and human podocytes controls a proteolytic programme that regulates cytoskeletal structure and cellular survival. J. Clin. Invest. 121, 3965–3980 (2011).
Schnabel, E., Anderson, J. M. & Farquhar, M. G. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J. Cell Biol. 111, 1255–1263 (1990).
Reiser, J., Kriz, W., Kretzler, M. & Mundel, P. The glomerular slit diaphragm is a modified adherens junction. J. Am. Soc. Nephrol. 11, 1–8 (2000).
Lehtonen, S., Lehtonen, E., Kudlicka, K., Holthofer, H. & Farquhar, M. G. Nephrin forms a complex with adherens junction proteins and CASK in podocytes and in Madin–Darby canine kidney cells expressing nephrin. Am. J. Pathol. 165, 923–936 (2004).
Fukasawa, H., Bornheimer, S., Kudlicka, K. & Farquhar, M. G. Slit diaphragms contain tight junction proteins. J. Am. Soc. Nephrol. 20, 1491–1503 (2009).
Sellin, L. et al. NEPH1 defines a novel family of podocin interacting proteins. FASEB J. 17, 115–117 (2003).
Yaoita, E. et al. Upregulation of connexin43 in glomerular podocytes in response to injury. Am. J. Pathol. 161, 1597–1606 (2002).
Pierchala, B. A., Munoz, M. R. & Tsui, C. C. Proteomic analysis of the slit diaphragm complex: CLIC5 is a protein critical for podocyte morphology and function. Kidney Int. 78, 868–882 (2010).
Reeves, W., Caulfield, J. P. & Farquhar, M. G. Differentiation of epithelial foot processes and filtration slits: sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab. Invest. 39, 90–100 (1978).
Quaggin, S. E. & Kreidberg, J. A. Development of the renal glomerulus: good neighbors and good fences. Development 135, 609–620 (2008).
Ruotsalainen, V. et al. Role of nephrin in cell junction formation in human nephrogenesis. Am. J. Pathol. 157, 1905–1916 (2000).
Goto, S. et al. Involvement of R-cadherin in the early stage of glomerulogenesis. J. Am. Soc. Nephrol. 9, 1234–1241 (1998).
Dahl, U. et al. Genetic dissection of cadherin function during nephrogenesis. Mol. Cell Biol. 22, 1474–1487 (2002).
Shono, A. et al. Podocin participates in the assembly of tight junctions between foot processes in nephrotic podocytes. J. Am. Soc. Nephrol. 18, 2525–2533 (2007).
Kurihara, H., Anderson, J. M., Kerjaschki, D. & Farquhar, M. G. The altered glomerular filtration slits seen in puromycin aminonucleoside nephrosis and protamine sulphate-treated rats contain the tight junction protein ZO-1. Am. J. Pathol. 141, 805–816 (1992).
Kramer-Zucker, A. G., Wiessner, S., Jensen, A. M. & Drummond, I. A. Organization of the pronephric filtration apparatus in zebrafish requires nephrin, podocin and the FERM domain protein mosaic eyes. Dev. Biol. 285, 316–329 (2005).
Zhang, F., Zhao, Y., Chao, Y., Muir, K. & Han, Z. Cubilin and amnionless mediate protein reabsorption in Drosophila nephrocytes. J. Am. Soc. Nephrol. 24, 209–216 (2013).
Weavers, H. et al. The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457, 322–326 (2009).
Zhuang, S. et al. Sns and Kirre, the Drosophila orthologues of nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development 136, 2335–2344 (2009).
Na, J. & Cagan, R. The Drosophila nephrocyte: back on stage. J. Am. Soc. Nephrol. 24, 161–163 (2013).
Helmstädter, M. et al. Functional study of mammalian Neph proteins in Drosophila melanogaster. PLoS ONE 7, e40300 (2012).
Simons, M. & Huber, T. B. Flying podocytes. Kidney Int. 75, 455–457 (2009).
Shen, K., Fetter, R. D. & Bargmann, C. I. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell 116, 869–881 (2004).
Neumann-Haefelin, E. et al. A model organism approach: defining the role of Neph proteins as regulators of neuron and kidney morphogenesis. Hum. Mol. Genet. 19, 2347–2359 (2010).
Wanner, N. et al. Functional and spatial analysis of C. elegans SYG-1 and SYG-2, orthologues of the Neph/nephrin cell adhesion module directing selective synaptogenesis. PLoS ONE 6, e23598 (2011).
Nagai, M. et al. Coxsackievirus and adenovirus receptor, a tight junction membrane protein, is expressed in glomerular podocytes in the kidney. Lab. Invest. 83, 901–911 (2003).
Lehtonen, S. et al. Cell junction-associated proteins IQGAP1, MAGI-2, CASK, spectrins, and α-actinin are components of the nephrin multiprotein complex. Proc. Natl Acad. Sci. USA 102, 9814–9819 (2005).
Hirabayashi, S. et al. MAGI-1 is a component of the glomerular slit diaphragm that is tightly associated with nephrin. Lab. Invest. 85, 1528–1543 (2005).
Kato, H. et al. Wnt/β-catenin pathway in podocytes integrates cell adhesion, differentiation, and survival. J. Biol. Chem. 286, 26003–26015 (2011).
Moeller, M. J. et al. Protocadherin FAT1 binds Ena/VASP proteins and is necessary for actin dynamics and cell polarization. EMBO J. 23, 3769–3779 (2004).
Inoue, T. et al. FAT is a component of glomerular slit diaphragms. Kidney Int. 59, 1003–1012 (2001).
Sawai, K. et al. Redistribution of connexin43 expression in glomerular podocytes predicts poor renal prognosis in patients with type 2 diabetes and overt nephropathy. Nephrol. Dial. Transplant. 21, 2472–2477 (2006).
Boerries, M. et al. Molecular fingerprinting of the podocyte reveals novel gene and protein regulatory networks. Kidney Int. 83, 1052–1064 (2013).
Verma, R. et al. Fyn binds to and phosphorylates the kidney slit diaphragm component nephrin. J. Biol. Chem. 278, 20716–20723 (2003).
Huber, T. B. et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signalling. Mol. Cell Biol. 23, 4917–4928 (2003).
Khoshnoodi, J. et al. Nephrin promotes cell–cell adhesion through homophilic interactions. Am. J. Pathol. 163, 2337–2346 (2003).
Gerke, P., Huber, T. B., Sellin, L., Benzing, T. & Walz, G. Homodimerization and heterodimerization of the glomerular podocyte proteins nephrin and Neph1. J. Am. Soc. Nephrol. 14, 918–926 (2003).
Barletta, G. M., Kovari, I. A., Verma, R. K., Kerjaschki, D. & Holzman, L. B. Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J. Biol. Chem. 278, 19266–19271 (2003).
Hartleben, B. et al. Neph–nephrin proteins bind the Par3–Par6-atypical protein kinase C (aPKC) complex to regulate podocyte cell polarity. J. Biol. Chem. 283, 23033–23038 (2008).
Huber, T. B. et al. Molecular basis of the functional podocin–nephrin complex: mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft microdomains. Hum. Mol. Genet. 12, 3397–3405 (2003).
Garg, P., Verma, R., Nihalani, D., Johnstone, D. B. & Holzman, L. B. Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol. Cell Biol. 27, 8698–8712 (2007).
Huang, M., Gu, G., Ferguson, E. L. & Chalfie, M. A stomatin-like protein necessary for mechanosensation in C. elegans. Nature 378, 292–295 (1995).
Huber, T. B. et al. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc. Natl Acad. Sci. USA 103, 17079–17086 (2006).
Schwarz, K. et al. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J. Clin. Invest. 108, 1621–1629 (2001).
Huber, T. B., Kottgen, M., Schilling, B., Walz, G. & Benzing, T. Interaction with podocin facilitates nephrin signalling. J. Biol. Chem. 276, 41543–41546 (2001).
Liu, G. et al. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J. Clin. Invest. 112, 209–221 (2003).
Palmen, T. et al. Interaction of endogenous nephrin and CD2-associated protein in mouse epithelial M-1 cell line. J. Am. Soc. Nephrol. 13, 1766–1772 (2002).
Rantanen, M. et al. Nephrin TRAP mice lack slit diaphragms and show fibrotic glomeruli and cystic tubular lesions. J. Am. Soc. Nephrol. 13, 1586–1594 (2002).
Miner, J. H. Life without nephrin: it's for the birds. J. Am. Soc. Nephrol. 23, 369–371 (2012).
Casotti, G. & Braun, E. J. Functional morphology of the glomerular filtration barrier of Gallus gallus. J. Morphol. 228, 327–334 (1996).
Dantzler, W. H. Significance of comparative studies for renal physiology. Am. J. Physiol. 238, F437–F444 (1980).
Endlich, N. & Endlich, K. The challenge and response of podocytes to glomerular hypertension. Semin. Nephrol. 32, 327–341 (2012).
Sachs, N. et al. Blood pressure influences end-stage renal disease of Cd151 knockout mice. J. Clin. Invest. 122, 348–358 (2012).
Dworkin, L. D., Hostetter, T. H., Rennke, H. G. & Brenner, B. M. Haemodynamic basis for glomerular injury in rats with desoxycorticosterone–salt hypertension. J. Clin. Invest. 73, 1448–1461 (1984).
Anderson, S., Meyer, T. W., Rennke, H. G. & Brenner, B. M. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J. Clin. Invest. 76, 612–619 (1985).
Moller, C. C., Flesche, J. & Reiser, J. Sensitizing the slit diaphragm with TrpC6 ion channels. J. Am. Soc. Nephrol. 20, 950–953 (2009).
Hartleben, B. et al. aPKCλ/ι and aPKCζ contribute to podocyte differentiation and glomerular maturation. J. Am. Soc. Nephrol. 24, 253–267 (2013).
Huber, T. B. et al. Loss of podocyte aPKCλ/ι causes polarity defects and nephrotic syndrome. J. Am. Soc. Nephrol. 20, 798–806 (2009).
Hartleben, B. et al. Role of the polarity protein Scribble for podocyte differentiation and maintenance. PLoS ONE 7, e36705 (2012).
Sugimoto, H. et al. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J. Biol. Chem. 278, 12605–12608 (2003).
Eremina, V. et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Invest. 111, 707–716 (2003).
Mattot, V. et al. Loss of the VEGF164 and VEGF188 isoforms impairs postnatal glomerular angiogenesis and renal arteriogenesis in mice. J. Am. Soc. Nephrol. 13, 1548–1560 (2002).
Foster, R. R. et al. Functional evidence that vascular endothelial growth factor may act as an autocrine factor on human podocytes. Am. J. Physiol. Renal Physiol. 284, F1263–F1273 (2003).
Foster, R. R., Saleem, M. A., Mathieson, P. W., Bates, D. O. & Harper, S. J. Vascular endothelial growth factor and nephrin interact and reduce apoptosis in human podocytes. Am. J. Physiol. Renal Physiol. 288, F48–F57 (2005).
Sison, K. et al. Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signalling. J. Am. Soc. Nephrol. 21, 1691–1701 (2010).
Jin, J. et al. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell 151, 384–399 (2012).
Quack, I. et al. PKC α mediates β-arrestin2-dependent nephrin endocytosis in hyperglycaemia. J. Biol. Chem. 286, 12959–12970 (2011).
Verma, R. et al. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J. Clin. Invest. 116, 1346–1359 (2006).
Lahdenpera, J. et al. Clustering-induced tyrosine phosphorylation of nephrin by Src family kinases. Kidney Int. 64, 404–413 (2003).
Kurihara, H., Anderson, J. M. & Farquhar, M. G. Increased Tyr phosphorylation of ZO-1 during modification of tight junctions between glomerular foot processes. Am. J. Physiol. 268, F514–F524 (1995).
Li, H., Lemay, S., Aoudjit, L., Kawachi, H. & Takano, T. SRC-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J. Am. Soc. Nephrol. 15, 3006–3015 (2004).
Zhu, J. et al. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 73, 556–566 (2008).
Zhang, S. Y. et al. c-mip impairs podocyte proximal signalling and induces heavy proteinuria. Sci. Signal. 3, ra39 (2010).
Audard, V. et al. Occurrence of minimal change nephrotic syndrome in classical Hodgkin lymphoma is closely related to the induction of c-mip in Hodgkin–Reed Sternberg cells and podocytes. Blood 115, 3756–3762 (2010).
Aoudjit, L. et al. Podocyte protein, nephrin, is a substrate of protein tyrosine phosphatase 1B. J. Signal Transduct. 2011, 376543 (2011).
Wharram, B. L. et al. Altered podocyte structure in GLEPP1 (Ptpro)-deficient mice associated with hypertension and low glomerular filtration rate. J. Clin. Invest. 106, 1281–1290 (2000).
Faul, C., Asanuma, K., Yanagida-Asanuma, E., Kim, K. & Mundel, P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 17, 428–437 (2007).
Faul, C. et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat. Med. 14, 931–938 (2008).
Jones, N. et al. Nck proteins maintain the adult glomerular filtration barrier. J. Am. Soc. Nephrol. 20, 1533–1543 (2009).
Jones, N. et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440, 818–823 (2006).
Schell, C. et al. N-WASP Is required for stabilization of podocyte foot processes. J. Am. Soc. Nephrol. 24, 713–721 (2013).
Harita, Y. et al. Phosphorylation of nephrin triggers Ca2+ signalling by recruitment and activation of phospholipase C-γ1. J. Biol. Chem. 284, 8951–8962 (2009).
Kanda, S. et al. Tyrosine phosphorylation-dependent activation of TrpC6 regulated by PLC-γ1 and nephrin: effect of mutations associated with focal segmental glomerulosclerosis. Mol. Biol. Cell 22, 1824–1835 (2011).
Vanhaesebroeck, B., Guillermet-Guibert, J., Graupera, M. & Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 11, 329–341 (2010).
Vanhaesebroeck, B., Stephens, L. & Hawkins, P. PI3K signalling: the path to discovery and understanding. Nat. Rev. Mol. Cell Biol. 13, 195–203 (2012).
Hara, K. et al. 1-Phosphatidylinositol 3-kinase activity is required for insulin-stimulated glucose transport but not for RAS activation in CHO cells. Proc. Natl Acad. Sci. USA 91, 7415–7419 (1994).
del Peso, L., Gonzalez-Garcia, M., Page, C., Herrera, R. & Nunez, G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278, 687–689 (1997).
Zhou, B. P. et al. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 3, 245–252 (2001).
Viglietto, G. et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27Kip1 by PKB/Akt-mediated phosphorylation in breast cancer. Nat. Med. 8, 1136–1144 (2002).
Cross, D. A., Alessi, D. R., Cohen, P., Andjelkovich, M. & Haemmings, B. A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 (1995).
Inoki, K., Li, Y., Zhu, T., Wu, J. & Guan, K. L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4, 648–657 (2002).
Paradis, S. & Ruvkun, G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 12, 2488–2498 (1998).
Kops, G. J. et al. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398, 630–634 (1999).
Takano, Y. et al. Transcriptional suppression of nephrin in podocytes by macrophages: roles of inflammatory cytokines and involvement of the PI3K/Akt pathway. FEBS Lett. 581, 421–426 (2007).
Qin, X. S. et al. Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J. Am. Soc. Nephrol. 20, 2534–2545 (2009).
Quack, I. et al. β-Arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc. Natl Acad. Sci. USA 103, 14110–14115 (2006).
Waters, A. M. et al. Notch promotes dynamin-dependent endocytosis of nephrin. J. Am. Soc. Nephrol. 23, 27–35 (2012).
Bechtel, W. et al. Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis. J. Am. Soc. Nephrol. 24, 727–743 (2013).
Soda, K. et al. Role of dynamin, synaptojanin, and endophilin in podocyte foot processes. J. Clin. Invest. 122, 4401–4411 (2012).
Volker, L. A. et al. Comparative analysis of Neph gene expression in mouse and chicken development. Histochem. Cell Biol. 137, 355–366 (2012).
Russo, L. M. et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int. 71, 504–513 (2007).
Arif, E. et al. Motor protein Myo1c is a podocyte protein that facilitates the transport of slit diaphragm protein Neph1 to the podocyte membrane. Mol. Cell Biol. 31, 2134–2150 (2011).
Mallik, L. et al. Solution structure analysis of cytoplasmic domain of podocyte protein Neph1 using small/wide angle x-ray scattering (SWAXS). J. Biol. Chem. 287, 9441–9453 (2012).
Greka, A. & Mundel, P. Cell biology and pathology of podocytes. Annu. Rev. Physiol. 74, 299–323 (2012).
George, B. & Holzman, L. B. Signalling from the podocyte intercellular junction to the actin cytoskeleton. Semin. Nephrol. 32, 307–318 (2012).
Kim, J. M. et al. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science 300, 1298–1300 (2003).
Acknowledgements
We apologize to all podocyte researchers whose interesting work and ideas could not be described in more detail owing to space limitations. The work in T. B. Huber's laboratory has been generously supported by grant number KFO208 from the DFG (German Research Foundation) to F. Grahammer and T. B. Huber, the Excellence Initiative of the German Federal and State Governments (grant numbers EXC 294 to T. B. Huber and GSC-4 (Spemann Graduate School of Biology and Medicine) to C. Schell and T. B. Huber), the Else Kröner Fresenius Stiftung (F. Grahammer, T. B. Huber), the Fritz Thyssen Stiftung (F. Grahammer, T. B. Huber) and the BMBF (Federal Ministry of Education and Research) Gerontosys II—NephAge (T. B. Huber).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to researching the data for the article, discussions of its content, writing the article and review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
T. B. Huber declares that he receives project-specific grant funding from Pfizer Pharma and has acted as a consultant for Abbott Pharma and Pfizer Pharma. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Grahammer, F., Schell, C. & Huber, T. The podocyte slit diaphragm—from a thin grey line to a complex signalling hub. Nat Rev Nephrol 9, 587–598 (2013). https://doi.org/10.1038/nrneph.2013.169
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2013.169
This article is cited by
-
Glomerular proteomic profiling reveals early differences between preexisting and de novo type 2 diabetes in human renal allografts
BMC Nephrology (2023)
-
New insights into the immune functions of podocytes: the role of complement
Molecular and Cellular Pediatrics (2023)
-
Efficient protocol for the differentiation of kidney podocytes from induced pluripotent stem cells, involving the inhibition of mTOR
Scientific Reports (2023)
-
Glycyrrhizic Acid Protects Glomerular Podocytes Induced by High Glucose by Modulating SNARK/AMPK Signaling Pathway
Current Medical Science (2023)
-
Expansion-enhanced super-resolution radial fluctuations enable nanoscale molecular profiling of pathology specimens
Nature Nanotechnology (2023)