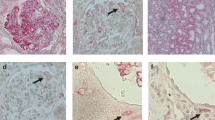
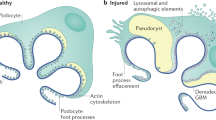
Key Points
-
Henoch–Schönlein purpura (HSP)—the most common vasculitis in children—is complicated by nephritis in about 30% of patients
-
Long-term prognosis of HSP nephritis depends mainly on the development of chronic kidney disease (CKD); often CKD risk cannot be predicted from the initial clinical and histological presentation
-
CKD can be observed at long-term follow-up even after apparent complete recovery from HSP nephritis
-
HSP nephritis and IgA nephropathy both result from glomerular deposition of aberrantly glycosylated IgA1 but have different histological features and clinical courses
-
Typically IgA nephropathy presents as slowly progressive mesangial lesions, whereas HSP nephritis presents as acute episodes characterized by inflammatory glomerular lesions that require prompt resolution to avoid chronic progression
-
Use of guidelines based on evidence obtained in adults with IgA nephropathy to select treatment for children with HSP nephritis risks delaying the provision of adequate therapies
Abstract
Henoch–Schönlein purpura (HSP) is the most common vasculitis in children, in whom prognosis is mostly dependent upon the severity of renal involvement. Nephritis is observed in about 30% of children with HSP. Renal damage eventually leads to chronic kidney disease in up to 20% of children with HSP nephritis in tertiary care centres, but in less than 5% of unselected patients with HSP, by 20 years after diagnosis. HSP nephritis and IgA nephropathy are related diseases resulting from glomerular deposition of aberrantly glycosylated IgA1. Although both nephritides present with similar histological findings and IgA abnormalities, they display pathophysiological differences with important therapeutic implications. HSP nephritis is mainly characterized by acute episodes of glomerular inflammation with endocapillary and mesangial proliferation, fibrin deposits and epithelial crescents that can heal spontaneously or lead to chronic lesions. By contrast, IgA nephropathy normally presents with slowly progressive mesangial lesions resulting from continuous low-grade deposition of macromolecular IgA1. This Review highlights the variable evolution of similar clinical and histological presentations among paediatric patients with HSP nephritis, which constitutes a challenge for their management, and discusses the treatment of these patients in light of current guidelines based on clinical evidence from adults with IgA nephropathy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Haycock, G. B. In Oxford Textbook of Clinical Nephrology 1st edn, Ch. 4 (eds Cameron, S. et al.) 595–612 (Oxford University Press, 1992).
Eleftheriou, D. & Brogan, P. A. Vasculitis in children. Best Pract. Res. Clin. Rheumatol. 23, 309–323 (2009).
Aalberse, J., Dolman, K., Ramnath, G., Pereira, R. R. & Davin, J. C. Henoch Schönlein purpura in children: an epidemiological study among Dutch paediatricians on incidence and diagnostic criteria. Ann. Rheum. Dis. 66, 1648–1650 (2007).
Dolezalova, P., Telekesova, P., Nemcova, D. & Hoza, J. Incidence of vasculitis in children in the Czech Republic: 2-year prospective epidemiology survey. J. Rheumatol. 31, 2295–2299 (2004).
Gardner-Medwin, J. M., Dolezalova, P., Cummins, C. & Southwood, T. R. Incidence of Henoch–Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet 360, 1197–1202 (2002).
Yang, Y. H. et al. A nationwide survey on epidemiological characteristics of childhood Henoch–Schönlein purpura in Taiwan. Rheumatology 44, 618–622 (2005).
Mills, J. A. et al. The American College of Rheumatology 1990 criteria for the classification of Henoch–Schönlein purpura. Arthritis Rheum. 33, 1114–1121 (1990).
Jennette, J. C. et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 37, 187–192 (1994).
Ozen, S. et al. EULAR/PRES endorsed consensus criteria for the classification of childhood vasculitides. Ann. Rheum. Dis. 65, 936–941 (2006).
Ozen, S. et al. EULAR/PRINTO/PRES criteria for Henoch–Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann. Rheum. Dis. 69, 798–806 (2010).
Lau, K. K. et al. Serum levels of galactose-deficient IgA in children with IgA nephropathy and Henoch–Schönlein purpura. Pediatr. Nephrol. 22, 2067–2072 (2007).
Davin, J. C., Ten Berge, I. J. & Weening, J. J. What is the difference between IgA nephropathy and Henoch–Schönlein purpura nephritis? Kidney Int. 59, 823–834 (2001).
Meadow, S. R. & Scott, D. G. Berger disease: Henoch–Schönlein without the rash. J. Pediatr. 106, 27–32 (1985).
White, R. H. R., Yoshikawa, N. & Feehally, J. In Pediatric Nephrology 4th edn, Ch. 41 (eds Barratt, T. M., Avner, E. D. & Harmon, W. E.) 691–706 (Williams & Wilkins, 1999).
Goldstein, A. R., White, R. H. R., Akuse, R. & Chantler, C. Long-term follow-up of childhood Henoch–Schönlein nephritis. Lancet 339, 280–282 (1992).
Koskimies, O., Mir, S., Rapola, J. & Vilska, J. Henoch–Schönlein nephritis: long-term prognosis of unselected patients. Arch. Intern. Med. 56, 482–484 (1981).
Bogdanovic, R. Henoch–Schönlein purpura nephritis in children: risk factors, prevention and treatment. Acta Paediatr. 98, 1882–1889 (2009).
Broyer, M. In Néphrologie Pédiatrique [French] 3rd edn, Ch. 2 (eds Royer, P. et al.) 75–98 (Flammarion Médecine-Sciences, 1983).
Jauhola, O. et al. Renal manifestations of Henoch–Schönlein purpura in a 6-month prospective study of 223 children. Arch. Dis. Child. 95, 877–882 (2010).
Narchi, H. Risk of long term renal impairment and duration of follow up recommended for Henoch–Schönlein purpura with normal or minimal urinary findings: a systematic review. Arch. Dis. Child. 90, 916–920 (2005).
Kaku, Y., Nohara, K. & Honda, S. Renal involvement in Henoch–Schönlein purpura: a multivariate analysis of prognostic factors. Kidney Int. 53, 1755–1759 (1998).
Coppo, R., Mazzucco, G., Cagnoli, L., Lupo, A. & Schena, F. P. Long-term prognosis of Henoch–Schönlein nephritis in adults and children. Italian Group of Renal Immunopathology Collaborative Study on Henoch–Schönlein purpura. Nephrol. Dial. Transplant. 12, 2277–2283 (1997).
Ronkainen, J., Nuutinen, M. & Koskimies, O. The adult kidney 24 years after childhood Henoch–Schönlein purpura: a retrospective cohort study. Lancet 360, 666–670 (2002).
Scharer, K. et al. Clinical outcome of Schönlein–Henoch purpura nephritis in children. Pediatr. Nephrol. 13, 816–823 (1999).
Counahan, R. et al. Prognosis of Henoch–Schönlein nephritis in children. Br. Med. J. 2, 11–14 (1977).
Bunchman, T. E., Mauer, S. M., Sibley, R. K. & Vernier, R. L. Anaphylactoid purpura: Characteristics of 16 patients who progressed to renal failure. Pediatr. Nephrol. 2, 393–397 (1988).
Niaudet, P., Murcia, I., Beaufils, H., Broyer, M. & Habib, R. Primary IgA nephropathies in children: prognosis and Treatment. Adv. Nephrol. Necker Hospital 22, 121–140 (1993).
Nussinovitch, N., Elishkevitz, K., Volovitz, B. & Nussinovitch, M. Hypertension as a late sequela of Henoch–Schönlein purpura. Clin. Pediatr. 44, 543–547 (2005).
Emancipator, S. N. In Pathology of the Kidney 4th edn, Ch. 6 (ed. Heptinstall, R. H.) 389–476 (Little Brown, 1993).
Haas, M. In Pathology of the Kidney 6th edn, Ch. 10 (eds Jennette, J. C. et al.) 423–486 (Lippincott, Williams & Wilkins, 2007).
Coppo, R. et al. Predictors of outcome in Henoch–Schönlein nephritis in children and adults. Am. J. Kidney Dis. 47, 993–1003 (2006).
Wakaki, H. et al. Henoch–Schönlein purpura nephritis with nephrotic state in children: predictors of poor outcomes. Pediatr. Nephrol. 26, 921–925 (2011).
Yoshikawa, N., White, R. H. & Cameron, A. H. Prognostic significance of the glomerular changes in Henoch–Schönlein nephritis. Clin. Nephrol. 16, 223–229 (1981).
Pillebout, E. et al. Henoch–Schönlein purpura in adults: outcome and prognostic factors. J. Am. Soc. Nephrol. 13, 1271–1278 (2002).
Fogazzi, G. B. et al. Long-term outcome of Schönlein–Henoch nephritis in the adult. Clin. Nephrol. 31, 60–66 (1989).
Meadow, S. R. et al. Schönlein–Henoch nephritis. Q. J. Med. 41, 241–258 (1972).
Heaton, J. M., Turner, D. R. & Cameron, J. S. Localization of glomerular “deposits” in Henoch–Schönlein nephritis. Histopathology 1, 93–104 (1977).
Schillinger, F. et al. Severe Schönlein–Henoch nephritis in adults: a report of twenty cases [French]. Nephrologie 21, 247–252 (2000).
Shenoy, M., Bradbury, M. G., Lewis, M. A. & Webb, N. J. Outcome of Henoch–Schönlein purpura nephritis treated with long-term immunosuppression. Pediatr. Nephrol. 22, 1717–1722 (2007).
Soylemezoglu, O. et al. Henoch–Schönlein nephritis: a nationwide study. Nephron Clin. Pract. 112, 199–204 (2009).
Shrestha, S. et al. Henoch Schönlein purpura with nephritis in adults: adverse prognostic indicators in a UK population. Q. J. Med. 99, 253–265 (2006).
Habib, R. In Néphrologie Pédiatrique 3rd edn, Ch. 11 (eds. Royer, P. et al.) 342–350 (Flammarion Médecine-Sciences, 1983).
Working Group of the International IgA Nephropathy Network and the Renal Pathology Society et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 76, 534–545 (2009).
Bennett, W. M. & Kincaid-Smith, P. Macroscopic hematuria in mesangial nephropathy: correlation with glomerular crescents and renal dysfunction. Kidney Int. 23, 392–400 (1983).
Foster, B. J., Bernard, C., Drummond, K. N. & Sharma, A. K. Effective therapy for severe Henoch–Schönlein purpura nephritis with prednisone and azathioprine: a clinical and histopathologic study. J. Pediatr. 136, 370–375 (2000).
Kanaan, N. et al. Recurrence and graft loss after kidney transplantation for Henoch–Schönlein purpura nephritis: a multicenter analysis. Clin. J. Am. Soc. Nephrol. 6, 1768–1772 (2011).
Thervet, E. et al. Histologic recurrence of Henoch–Schönlein purpura nephropathy after renal transplantation on routine allograft biopsy. Transplantation 92, 907–912 (2011).
Ponticelli, C. et al. Kidney transplantation in patients with IgA mesangial glomerulonephritis. Kidney Int. 60, 1948–1954 (2001).
Briganti, E. M., Russ, G. R., McNeil, J. J., Atkins, R. C. & Chadban, S. J. Risk of renal allograft loss from recurrent glomerulonephritis. N. Engl. J. Med. 347, 103–109 (2002).
Kincaid-Smith, P., Nicholls, K. & Birchall, I. Polymorphs infiltrate glomeruli in mesangial IgA glomerulonephritis. Kidney Int. 36, 1108–1111 (1989).
Cunningham, M. A., Kitching, A. R., Tipping, P. G. & Holdsworth, S. R. Fibrin independent proinflammatory effects of tissue factor in experimental crescentic glomerulonephritis. Kidney Int. 66, 647–654 (2004).
Kamitsuji, H. et al. Urinary cross-linked fibrin degradation products in glomerular disease with crescents. Clin. Nephrol. 29, 124–128 (1988).
Szeto, C. C. et al. Grading of acute and chronic renal lesions in Henoch–Schönlein purpura. Mol. Pathol. 14, 635–640 (2001).
Vassalli, P. & McCluskey, R. T. The pathogenetic role of the coagulation process in rabbit Masugi nephritis. Am. J. Pathol. 45, 653–677 (1964).
Hisano, S., Matsushita, M., Fujita, T. & Iwasaki, H. Activation of the lectin complement pathway in Henoch–Schönlein purpura nephritis. Am. J. Kidney. Dis. 45, 295–302 (2005).
Novak, J., Julian, B. A., Mestecky, J. & Renfrow, M. B. Glycosylation of IgA1 and pathogenesis of IgA nephropathy. Semin. Immunopathol. 34, 365–382 (2012).
Allen, A. C., Willis, F. R., Beattie, T. J. & Feehally, J. Abnormal IgA glycosylation in Henoch–Schönlein purpura restricted to patients with clinical nephritis. Nephrol. Dial. Transplant. 13, 930–934 (1998).
Serino, G., Sallustio, F., Cox, S. N., Pesce, F. & Schena, F. P. Abnormal miR-148b expression promotes aberrant glycosylation of IgA1 in IgA nephropathy. J. Am. Soc. Nephrol. 23, 814–824 (2012).
Chintalacharuvu, S. R. et al. T cell cytokines determine the severity of experimental IgA nephropathy by regulating IgA glycosylation. Clin. Exp. Immunol. 126, 326–333 (2001).
Chintalacharuvu, S. R. et al. T cell cytokine polarity as a determinant of immunoglobulin A (IgA) glycosylation and the severity of experimental IgA nephropathy. Clin. Exp. Immunol. 153, 456–462 (2008).
Inoshita, H. et al. Disruption of SMAD4 expression in T cells leads to IgA nephropathy-like manifestations. PLoS ONE 8, e78736 (2013).
Yamada, K. et al. Down-regulation of core 1 β1, 3-galactosyltransferase and Cosmc by TH2 cytokine alters O-glycosylation of IgA1 . Nephrol. Dial. Transplant. 25, 3890–3897 (2010).
Suzuki, H. et al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J. Clin. Invest. 119, 1668–1677 (2009).
Wyatt, R. J. & Julian, B. A. IgA nephropathy. N. Engl. J. Med. 368, 2402–2414 (2013).
Smith, A. C., Molyneux, K., Feehally, J. & Barratt, J. O-glycosylation of serum IgA1 antibodies against mucosal and systemic antigens in IgA nephropathy. J. Am. Soc. Nephrol. 17, 3520–3528 (2006).
Davin, J. C., Forget, P. & Mahieu, P. R. Increased intestinal permeability to 51Cr EDTA is correlated with IgA immune complex-plasma levels in children with IgA-associated nephropathies. Acta Paediatr. Scand. 77, 118–124 (1988).
de Fijter, J. W. et al. Deficient IgA1 immune response to nasal cholera toxin subunit B in primary IgA nephropathy. Kidney Int. 50, 952–961 (1996).
Monteiro, R. C. The role of IgA and IgA Fc receptors in inflammation. J. Clin. Immunol. 30, 1–9 (2010).
Vuong, M. T. et al. Association of soluble CD89 levels with disease progression but not susceptibility in IgA nephropathy. Kidney Int. 78, 1281–1287 (2010).
Davin, J. C. et al. Possible pathogenic role of IgE in Henoch–Schönlein purpura. Pediatr. Nephrol. 8, 169–171 (1994).
Kawasaki, Y., Hosoya, M. & Suzuki, H. Possible pathologenic role of interleukin-5 and eosino cationic protein in Henoch–Schönlein purpura nephritis. Pediatr. Int. 47, 512–517 (2005).
Namgoong, M. K., Lim, B. K. & Kim, J. S. Eosinophil cationic protein in Henoch–Schönlein purpura and in IgA nephropathy. Pediatr. Nephrol. 11, 703–706 (1997).
Chen, A., Chen, W. P., Sheu, L. F. & Lin, C. Y. Pathogenesis of IgA nephropathy: in vitro activation of human mesangial cells by IgA. J. Pathol. 173, 119–126 (1994).
Gomez-Guerrero, C., Lopez-Armada, M. J., Gonzalez, E. & Egido, J. Soluble IgA and IgG aggregates are catabolized by cultured rat mesangial cells and induce production of TNF-α and IL-6, and proliferation. J. Immunol. 153, 5247–5255 (1994).
Oortwijn, B. D. et al. Differential glycosylation of polymeric and monomeric IgA: a possible role in glomerular inflammation in IgA nephropathy. J. Am. Soc. Nephrol. 17, 3529–3539 (2006).
Suzuki, H. et al. The pathophysiology of IgA nephropathy. J. Am. Soc. Nephrol. 22, 1795–1803 (2011).
Yan, Y., Xu, L. X., Zhang, J. J., Zhang, Y. & Zhao, M. H. Self-aggregated deglycosylated IgA1 with or without IgG were associated with the development of IgA nephropathy. Clin. Exp. Immunol. 144, 17–24 (2006).
Schlöndorff, D. & Banas, B. The mesangial cell revisited: no cell is an island. J. Am. Soc. Nephrol. 20, 1179–1187 (2009).
Wan, J. X. et al. Complement 3 is involved in changing the phenotype of human glomerular mesangial cells. J. Cell Physiol. 213, 495–501 (2007).
Camilla, R. et al. Oxidative stress and galactose-deficient IgA1 as markers of progression in IgA nephropathy. Clin. J. Am. Soc. Nephrol. 6, 1903–1911 (2011).
Wang, C. et al. Mesangial cells stimulated by immunoglobin A1 from IgA nephropathy upregulate transforming growth factor β1 synthesis in podocytes via renin–angiotensin system activation. Arch. Med. Res. 41, 255–260 (2010).
Coppo, R. et al. Aberrantly glycosylated IgA1 induces mesangial cells to produce platelet-activating factor that mediates nephrin loss in cultured podocytes. Kidney Int. 77, 417–427 (2010).
Horii, Y. et al. Involvement of IL-6 in mesangial proliferative glomerulonephritis. J. Immunol. 143, 3949–3955 (1989).
Kashem, A. et al. Glomerular FcαR expression and disease activity in IgA nephropathy. Am. J. Kidney Dis. 30, 389–396 (1997).
Niemir, Z. I. et al. PDGF and TGF-β contribute to the natural course of human IgA glomerulonephritis. Kidney Int. 48, 1530–1541 (1995).
Lopez-Armada, M. J., Gomez-Guerrero, C. & Egido, J. Receptors for immune complexes activate gene expression and synthesis of matrix proteins in cultured rat and human mesangial cells: role of TGF-β. J. Immunol. 157, 2136–2142 (1996).
Terada, Y. et al. Expression of PDGF and PDGF receptor mRNA in IgA nephropathy. J. Am. Soc. Nephrol. 8, 817–819 (1997).
Yokoyama, H. et al. Urinary levels of chemokines (MCAF/MCP-1, IL-8) reflect distinct disease activities and phases of human IgA nephropathy. J. Leukocyte Biol. 63, 493–499 (1998).
Camussi, G. et al. Tumor necrosis factor induces contraction of mesangial cells and alters their cytoskeletons. Kidney Int. 38, 795–802 (1990).
Ha, T. S. The role of tumor necrosis factor-α in Henoch–Schönlein purpura. Pediatr. Nephrol. 20, 149–153 (2005).
Couser, W. G. Glomerulonephritis. Lancet 353, 1509–1515 (1999).
Jennette, J. C. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 63, 1164–1177 (2003).
Bonsib, S. Glomerular basement membrane discontinuities: scanning electron microscopic study of acellular glomeruli. Am. J. Pathol. 119, 357–360 (1985).
Atkins, R. C., Nikolic-Paterson, D. J., Song, Q. & Lan, H. Y. Modulators of crescentic glomerulonephritis. J. Am. Soc. Nephrol. 7, 2271–2278 (1996).
Lan, H. Y., Nikolic-Paterson, D. J. & Atkins, R. C. Involvement of activated periglomerular leukocytes in the rupture of Bowman's capsule and crescent progression in experimental glomerulonephritis. Lab. Invest. 67, 743–751 (1992).
Lan, H. Y., Nikolic-Paterson, D. J., Mu, W., Vannice, J. L. & Atkins, R. C. Interleukin-1 receptor antagonist halts the progression of established crescentic glomerulonephritis in the rat. Kidney Int. 47, 1303–1309 (1995).
Isaka, Y. et al. Gene therapy by transforming growth factor-β receptor–IgG Fc chimera suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int. 55, 465–475 (1999).
Adler, S. & Brady, H. R. Cell adhesion molecules and the glomerulopathies. Am. J. Med. 107, 371–386 (1999).
Morel-Maroger Striker, L., Killen, P. D., Chi, E. & Striker, G. E. The composition of glomerulosclerosis. I. Studies in focal sclerosis, crescentic glomerulonephritis, and membranoproliferative glomerulonephritis. Lab. Invest. 51, 181–192 (1984).
Mathieson, P. W. The ins and outs of glomerular crescent formation. Clin. Exp. Immunol. 110, 155–157 (1997).
Emancipator, S. N. IgA nephropathy: morphologic expression and pathogenesis. Am. J. Kidney. Dis. 23, 451–462 (1994).
Border, W. A. & Noble, N. A. Transforming growth factor in tissue fibrosis. N. Engl. J. Med. 331, 1286–1292 (1994).
Lin, Q. et al. Henoch–Schönlein purpura with hypocomplementemia. Pediatr. Nephrol. 27, 801–806 (2012).
Roos, A. et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J. Am. Soc. Nephrol. 17, 1724–1734 (2006).
Espinosa, M. et al. Mesangial C4d deposition: a new prognostic factor in IgA nephropathy. Nephrol. Dial. Transplant. 24, 886–891 (2009).
McLean, R. H., Michael, A. F., Fish, A. J. & Vernier, R. L. In Pediatric Nephrology 1st edn, Ch. 25 (eds Rubin, M. I. & Barratt, T. M.) 584–588 (Williams & Wilkins, 1975).
Coppo, R. & Amore, A. In Pediatric Nephrology 5th edn, Ch. 25 (eds Avner, E. D. et al.) 851–864 (Lippincott Williams & Wilkins, 2004).
Rees, L., Webb, N. J. A. & Brogan, P. A. In Paediatric Nephrology 2nd edn, Ch. 20 (eds Rees, L. et al.) 312–313 (Oxford University Press, 2007).
Tarshish, P., Bernstein, J. & Edelmann, C. M. Jr. Henoch–Schönlein purpura nephritis: course of disease and efficacy of cyclophosphamide. Pediatr. Nephrol. 19, 51–56 (2004).
Jauhola, O. et al. Cyclosporine A vs. methylprednisolone for Henoch–Schönlein nephritis: a randomized trial. Pediatr. Nephrol. 26, 2159–2166 (2011).
Pillebout, E. et al. Addition of cyclophosphamide to steroids provides no benefit compared with steroids alone in treating adult patients with severe Henoch Schönlein purpura. Kidney Int. 78, 495–502 (2010).
Kidney Disease: Improving Global Outcomes. Chapter 11: Henoch–Schönlein purpura nephritis. Kidney Int. Suppl. 2, 218–220 (2012).
Davin, J. C. Henoch–Schönlein purpura nephritis: pathophysiology, treatment, and future strategy. Clin. J. Am. Soc. Nephrol. 6, 679–689 (2011).
Hattori, M. et al. Plasmapheresis as the sole therapy for rapidly progressive Henoch–Schönlein purpura nephritis in children. Am. J. Kidney Dis. 33, 427–433 (1999).
Shenoy, M., Ognjanovic, M. V. & Coulthard, M. G. Treating severe Henoch–Schönlein and IgA nephritis with plasmapheresis alone. Pediatr. Nephrol. 22, 1167–1171 (2007).
Niaudet, P. & Habib, R. Methylprednisolone pulse therapy in the treatment of severe forms of Schönlein–Henoch purpura nephritis. Pediatr. Nephrol. 12, 238–243 (1998).
Andersen, R. F., Rubak, S., Jespersen, B. & Rittig, S. Early high dose immunosuppression in Henoch–Schönlein nephrotic syndrome may improve outcome. Scand. J. Urol. Nephrol. 43, 409–415 (2009).
Flynn, J. T., Smoyer, W. E., Bunchman, T. E., Kershaw, D. B. & Sedman, A. B. Treatment of Henoch–Schönlein purpura glomerulonephritis in children with high-dose corticosteroids plus oral cyclophosphamide. Am. J. Nephrol. 21, 128–133 (2001).
Kawasaki, Y., Suyama, K., Hashimoto, K. & Hosoya, M. Methylprednisolone pulse plus mizoribine in children with Henoch–Schönlein purpura nephritis. Clin. Rheumatol. 30, 529–535 (2011).
Kawasaki, Y., Suzuki, J. & Suzuki, H. Efficacy of methylprednisolone and urokinase pulse therapy combined with or without cyclophosphamide in severe Henoch–Schönlein nephritis: a clinical and histopathological study. Nephrol. Dial. Transplant. 19, 858–864 (2004).
Kawasaki, Y., Suzuki, J., Nozawa, R., Suzuki, S. & Suzuki, H. Efficacy of methylprednisolone and urokinase pulse therapy for severe Henoch–Schönlein nephritis. Pediatrics 111, 785–789 (2003).
Ashton, H., Frenk, E. & Stevenson, C. J. Therapeutics. XV. The management of Henoch–Schönlein purpura. Br. J. Dermatol. 85, 199–203 (1971).
Borges, W. H. Anaphylactoid purpura. Med. Clin. North Am. 56, 201–206 (1972).
Ou, Z. L. et al. Effective methylprednisolone dose in experimental crescentic glomerulonephritis. Am. J. Kidney Dis. 37, 411–417 (2001).
Lameire, N. H. et al. Acute kidney injury: an increasing global concern. Lancet 382, 170–179 (2013).
Branten, A. J., Kock-Jansen, M., Klasen, I. S. & Wetzels, J. F. Urinary excretion of complement C3d in patients with renal diseases. Eur. J. Clin. Invest. 33, 449–456 (2003).
Wickman, L. et al. Urine podocyte mRNAs, proteinuria, and progression in human glomerular diseases. J. Am. Soc. Nephrol. 24, 2081–2095 (2013).
Delanghe, S. E. et al. Soluble transferrin receptor in urine, a new biomarker for IgA nephropathy and Henoch–Schönlein purpura nephritis. Clin. Biochem. 46, 591–597 (2013).
Moura, I. C. et al. Identification of the transferrin receptor as a novel immunoglobulin (Ig)A1 receptor and its enhanced expression on mesangial cells in IgA nephropathy. J. Exp. Med. 194, 417–425 (2001).
Park, S. J. et al. Advances in our understanding of the pathogenesis of Henoch–Schönlein purpura and the implications for improving its diagnosis. Expert Rev. Clin. Immunol. 9, 1223–1238 (2013).
Ninchoji, T. et al. Treatment strategies for Henoch–Schönlein purpura nephritis by histological and clinical severity. Pediatr. Nephrol. 26, 563–569 (2011).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to researching the data for the article, discussions of its content, writing the article and review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Davin, JC., Coppo, R. Henoch–Schönlein purpura nephritis in children. Nat Rev Nephrol 10, 563–573 (2014). https://doi.org/10.1038/nrneph.2014.126
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2014.126
This article is cited by
-
Regulatory T and B cells in pediatric Henoch–Schönlein purpura: friends or foes?
Arthritis Research & Therapy (2024)
-
The Association of HMGB1 and RAGE Gene Polymorphisms with IgA Vasculitis
Biochemical Genetics (2023)
-
Risk factors associated with short- and long-term kidney injury in children with post infectious glomerulonephritis
Pediatric Nephrology (2023)
-
Kidney biopsy diagnosis in childhood in the Norwegian Kidney Biopsy Registry and the long-term risk of kidney replacement therapy: a 25-year follow-up
Pediatric Nephrology (2023)
-
Long-term outcome of combination therapy with corticosteroids, mizoribine and RAS inhibitors as initial therapy for severe childhood IgA vasculitis with nephritis
Pediatric Nephrology (2023)