Key Points
-
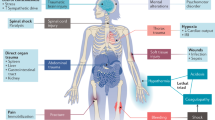
Various components of the innate and adaptive immune systems are implicated in the pathogenesis and repair of acute kidney injury (AKI)
-
The roles of individual immune cell types have been most thoroughly investigated in models of ischaemic AKI
-
Various immune cells traffic into the post-ischaemic kidney and show changes in phenotypes and numbers depending on the time course after establishment of ischaemic AKI
-
The roles of macrophages, renal dendritic cells and T regulatory cells differ according to the pathogenesis of AKI
-
Although numerous studies in animal models of AKI show therapeutic potential for modulating immune cells, big hurdles must be overcome before applying these findings to patients
-
Functions and interactions of specific immune cell types and humoral factors in AKI differ between human disease and animal models, and depend on the type and stage of injury
Abstract
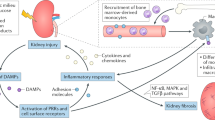
Acute kidney injury (AKI) prolongs hospital stay and increases mortality in various clinical settings. Ischaemia–reperfusion injury (IRI), nephrotoxic agents and infection leading to sepsis are among the major causes of AKI. Inflammatory responses substantially contribute to the overall renal damage in AKI. Both innate and adaptive immune systems are involved in the inflammatory process occurring in post-ischaemic AKI. Proinflammatory damage-associated molecular patterns, hypoxia-inducible factors, adhesion molecules, dysfunction of the renal vascular endothelium, chemokines, cytokines and Toll-like receptors are involved in the activation and recruitment of immune cells into injured kidneys. Immune cells of both the innate and adaptive immune systems, such as neutrophils, dendritic cells, macrophages and lymphocytes contribute to the pathogenesis of renal injury after IRI, and some of their subpopulations also participate in the repair process. These immune cells are also involved in the pathogenesis of nephrotoxic AKI. Experimental studies of immune cells in AKI have resulted in improved understanding of the immune mechanisms underlying AKI and will be the foundation for development of novel diagnostic and therapeutic targets. This Review describes what is currently known about the function of the immune system in the pathogenesis and repair of ischaemic and nephrotoxic AKI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Thadhani, R., Pascual, M. & Bonventre, J. V. Acute renal failure. N. Engl. J. Med. 334, 1448–1460 (1996).
Coca, S. G., Singanamala, S. & Parikh, C. R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 81, 442–448 (2012).
Goncalves, G. M., Zamboni, D. S. & Camara, N. O. The role of innate immunity in septic acute kidney injuries. Shock 34, S22–S26 (2010).
Halazun, K. J., Al-Mukhtar, A., Aldouri, A., Willis, S. & Ahmad, N. Warm ischemia in transplantation: search for a consensus definition. Transplant. Proc. 39, 1329–1331 (2007).
Jang, H. R., Ko, G. J., Wasowska, B. A. & Rabb, H. The interaction between ischemia-reperfusion and immune responses in the kidney. J. Mol. Med. 87, 859–864 (2009).
Jang, H. R. & Rabb, H. The innate immune response in ischemic acute kidney injury. Clin. Immunol. 130, 41–50 (2009).
Kurts, C., Panzer, U., Anders, H. J. & Rees, A. J. The immune system and kidney disease: basic concepts and clinical implications. Nat. Rev. Immunol. 13, 738–753 (2013).
Wolfs, T. G. et al. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-γ and TNF-α mediated up-regulation during inflammation. J. Immunol. 168, 1286–1293 (2002).
Kim, B. S. et al. Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation 79, 1370–1377 (2005).
Kaissling, B. & Le Hir, M. Characterization and distribution of interstitial cell types in the renal cortex of rats. Kidney Int. 45, 709–720 (1994).
Kruger, T. et al. Identification and functional characterization of dendritic cells in the healthy murine kidney and in experimental glomerulonephritis. J. Am. Soc. Nephrol. 15, 613–621 (2004).
Soos, T. J. et al. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 70, 591–596 (2006).
Woltman, A. M. et al. Quantification of dendritic cell subsets in human renal tissue under normal and pathological conditions. Kidney Int. 71, 1001–1008 (2007).
Nelson, P. J. et al. The renal mononuclear phagocytic system. J. Am. Soc. Nephrol. 23, 194–203 (2012).
Guilliams, M. et al. From skin dendritic cells to a simplified classification of human and mouse dendritic cell subsets. Eur. J. Immunol. 40, 2089–2094 (2010).
Miller, J. C. et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat. Immunol. 13, 888–899 (2012).
Kim, K. W. et al. In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood 118, e156–e167 (2011).
Tittel, A. P. et al. Functionally relevant neutrophilia in CD11c diphtheria toxin receptor transgenic mice. Nat. Methods 9, 385–390 (2012).
Timoshanko, J. R., Kitching, A. R., Semple, T. J., Tipping, P. G. & Holdsworth, S. R. A pathogenetic role for mast cells in experimental crescentic glomerulonephritis. J. Am. Soc. Nephrol. 17, 150–159 (2006).
Scandiuzzi, L. et al. Mouse mast cell protease-4 deteriorates renal function by contributing to inflammation and fibrosis in immune complex-mediated glomerulonephritis. J. Immunol. 185, 624–633 (2010).
Gan, P. Y. et al. Mast cells contribute to peripheral tolerance and attenuate autoimmune vasculitis. J. Am. Soc. Nephrol. 23, 1955–1966 (2012).
Ascon, D. B. et al. Normal mouse kidneys contain activated and CD3+CD4− CD8− double-negative T lymphocytes with a distinct TCR repertoire. J. Leukoc. Biol. 84, 1400–1409 (2008).
Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045 (1994).
Matzinger, P. The danger model: a renewed sense of self. Science 296, 301–305 (2002).
Rock, K. L., Latz, E., Ontiveros, F. & Kono, H. The sterile inflammatory response. Annu. Rev. Immunol. 28, 321–342 (2010).
Kono, H., Chen, C. J., Ontiveros, F. & Rock, K. L. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J. Clin. Invest. 120, 1939–1949 (2010).
Imaeda, A. B. et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J. Clin. Invest. 119, 305–314 (2009).
Eigenbrod, T., Park, J. H., Harder, J., Iwakura, Y. & Nunez, G. Cutting edge: critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1 α released from dying cells. J. Immunol. 181, 8194–8198 (2008).
Chen, S. W. et al. Mice that overexpress human heat shock protein 27 have increased renal injury following ischemia reperfusion. Kidney Int. 75, 499–510 (2009).
Thurman, J. M. Triggers of inflammation after renal ischemia/reperfusion. Clin. Immunol. 123, 7–13 (2007).
Rosenberger, C. et al. Cellular responses to hypoxia after renal segmental infarction. Kidney Int. 64, 874–886 (2003).
Matsumoto, M. et al. Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J. Am. Soc. Nephrol. 14, 1825–1832 (2003).
Bernhardt, W. M. et al. Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J. Am. Soc. Nephrol. 17, 1970–1978 (2006).
Kelly, K. J. et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J. Clin. Invest. 97, 1056–1063 (1996).
Takada, M., Nadeau, K. C., Shaw, G. D., Marquette, K. A. & Tilney, N. L. The cytokine-adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney. Inhibition by a soluble P-selectin ligand. J. Clin. Invest. 99, 2682–2690 (1997).
Brodsky, S. V. et al. Endothelial dysfunction in ischemic acute renal failure: rescue by transplanted endothelial cells. Am. J. Physiol. Renal Physiol. 282, F1140–F1149 (2002).
Sutton, T. A. et al. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am. J. Physiol. Renal Physiol. 285, F191–F198 (2003).
Liu, M. et al. Effect of T cells on vascular permeability in early ischemic acute kidney injury in mice. Microvasc. Res. 77, 340–347 (2009).
Eickelberg, O. et al. Functional activation of heat shock factor and hypoxia-inducible factor in the kidney. J. Am. Soc. Nephrol. 13, 2094–2101 (2002).
Cao, C. C. et al. In vivo transfection of NF-kappaB decoy oligodeoxynucleotides attenuate renal ischemia/reperfusion injury in rats. Kidney Int. 65, 834–845 (2004).
Donnahoo, K. K. et al. Early kidney TNF-α expression mediates neutrophil infiltration and injury after renal ischemia-reperfusion. Am. J. Physiol. 277, R922–R929 (1999).
Daha, M. R. & van Kooten, C. Is the proximal tubular cell a proinflammatory cell? Nephrol. Dial. Transplant. 15, S41–S43 (2000).
Hiroyoshi, T. et al. Splenectomy protects the kidneys against ischemic reperfusion injury in the rat. Transpl. Immunol. 27, 8–11 (2012).
Anders, H. J., Vielhauer, V. & Schlondorff, D. Chemokines and chemokine receptors are involved in the resolution or progression of renal disease. Kidney Int. 63, 401–415 (2003).
Swaminathan, S. & Griffin, M. D. First responders: understanding monocyte-lineage traffic in the acutely injured kidney. Kidney Int. 74, 1509–1511 (2008).
Miura, M., Fu, X., Zhang, Q. W., Remick, D. G. & Fairchild, R. L. Neutralization of Gro α and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am. J. Pathol. 159, 2137–2145 (2001).
Araki, M. et al. Expression of IL-8 during reperfusion of renal allografts is dependent on ischemic time. Transplantation 81, 783–788 (2006).
Fiorina, P. et al. Role of CXC chemokine receptor 3 pathway in renal ischemic injury. J. Am. Soc. Nephrol. 17, 716–723 (2006).
Furuichi, K. et al. CCR2 signaling contributes to ischemia-reperfusion injury in kidney. J. Am. Soc. Nephrol. 14, 2503–2515 (2003).
Furuichi, K., Gao, J. L. & Murphy, P. M. Chemokine receptor CX3CR1 regulates renal interstitial fibrosis after ischemia-reperfusion injury. Am. J. Pathol. 169, 372–387 (2006).
Wu, H. et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J. Clin. Invest. 117, 2847–2859 (2007).
Allam, R. et al. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J. Am. Soc. Nephrol. 23, 1375–1388 (2012).
Wu, H. et al. HMGB1 contributes to kidney ischemia reperfusion injury. J. Am. Soc. Nephrol. 21, 1878–1890 (2010).
Leemans, J. C. et al. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J. Clin. Invest. 115, 2894–2903 (2005).
Iyer, S. S. et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl Acad. Sci. USA 106, 20388–20393 (2009).
Chiao, H. et al. α-melanocyte-stimulating hormone protects against renal injury after ischemia in mice and rats. J. Clin. Invest. 99, 1165–1172 (1997).
Nemoto, T. et al. Small molecule selectin ligand inhibition improves outcome in ischemic acute renal failure. Kidney Int. 60, 2205–2214 (2001).
Solez, K., Morel–Maroger, L. & Sraer, J. D. The morphology of “acute tubular necrosis” in man: analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine (Baltimore) 58, 362–376 (1979).
Friedewald, J. J. & Rabb, H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 66, 486–491 (2004).
Li, L. et al. IL-17 produced by neutrophils regulates IFN-γ-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J. Clin. Invest. 120, 331–342 (2010).
Fukuzawa, N. et al. High renal ischemia temperature increases neutrophil chemoattractant production and tissue injury during reperfusion without an identifiable role for CD4 T cells in the injury. Transpl. Immunol. 22, 62–71 (2009).
Thornton, M. A., Winn, R., Alpers, C. E. & Zager, R. A. An evaluation of the neutrophil as a mediator of in vivo renal ischemic-reperfusion injury. Am. J. Pathol. 135, 509–515 (1989).
Rabb, H. et al. Role of CD11a and CD11b in ischemic acute renal failure in rats. Am. J. Physiol. 267, F1052–F1058 (1994).
Hayama, T. et al. Benefical effect of neutrophil elastase inhibitor on renal warm ischemia-reperfusion injury in the rat. Transplant. Proc. 38, 2201–2202 (2006).
Roelofs, J. J. et al. Tissue-type plasminogen activator modulates inflammatory responses and renal function in ischemia reperfusion injury. J. Am. Soc. Nephrol. 17, 131–140 (2006).
Mizuno, S. & Nakamura, T. Prevention of neutrophil extravasation by hepatocyte growth factor leads to attenuations of tubular apoptosis and renal dysfunction in mouse ischemic kidneys. Am. J. Pathol. 166, 1895–1905 (2005).
Rouschop, K. M. et al. Protection against renal ischemia reperfusion injury by CD44 disruption. J. Am. Soc. Nephrol. 16, 2034–2043 (2005).
Haug, C. E. et al. A phase I trial of immunosuppression with anti-ICAM-1 (CD54) mAb in renal allograft recipients. Transplantation 55, 766–772 (1993).
Salmela, K. et al. A randomized multicenter trial of the anti-ICAM-1 monoclonal antibody (enlimomab) for the prevention of acute rejection and delayed onset of graft function in cadaveric renal transplantation: a report of the European Anti-ICAM-1 Renal Transplant Study Group. Transplantation 67, 729–736 (1999).
Riera, M. et al. Neutrophils accentuate renal cold ischemia-reperfusion injury. Dose-dependent protective effect of a platelet-activating factor receptor antagonist. J. Pharmacol. Exp. Ther. 280, 786–794 (1997).
Vinten–Johansen, J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc. Res. 61, 481–497 (2004).
Crinnion, J. N., Homer-Vanniasinkam, S. & Gough, M. J. Skeletal muscle reperfusion injury: pathophysiology and clinical considerations. Cardiovasc. Surg. 1, 317–324 (1993).
Ysebaert, D. K. et al. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol. Dial. Transplant. 15, 1562–1574 (2000).
De Greef, K. E. et al. Anti-B7-1 blocks mononuclear cell adherence in vasa recta after ischemia. Kidney Int. 60, 1415–1427 (2001).
Celie, J. W. et al. Subendothelial heparan sulfate proteoglycans become major L-selectin and monocyte chemoattractant protein-1 ligands upon renal ischemia/reperfusion. Am. J. Pathol. 170, 1865–1878 (2007).
Jo, S. K., Sung, S. A., Cho, W. Y., Go, K. J. & Kim, H. K. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol. Dial. Transplant. 21, 1231–1239 (2006).
He, Z. et al. Macrophages are not the source of injurious interleukin-18 in ischemic acute kidney injury in mice. Am. J. Physiol. Renal Physiol. 296, F535–F542 (2009).
Day, Y. J., Huang, L., Ye, H., Linden, J. & Okusa, M. D. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am. J. Physiol. Renal Physiol. 288, F722–F731 (2005).
Gueler, F. et al. Statins attenuate ischemia-reperfusion injury by inducing heme oxygenase-1 in infiltrating macrophages. Am. J. Pathol. 170, 1192–1199 (2007).
Persy, V. P., Verhulst, A., Ysebaert, D. K., De Greef, K. E. & De Broe, M. E. Reduced postischemic macrophage infiltration and interstitial fibrosis in osteopontin knockout mice. Kidney Int. 63, 543–553 (2003).
Ko, G. J., Boo, C. S., Jo, S. K., Cho, W. Y. & Kim, H. K. Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol. Dial. Transplant. 23, 842–852 (2008).
Vinuesa, E. et al. Macrophage involvement in the kidney repair phase after ischaemia/reperfusion injury. J. Pathol. 214, 104–113 (2008).
Huen, S. C., Moeckel, G. W. & Cantley, L. G. Macrophage-specific deletion of transforming growth factor-β1 does not prevent renal fibrosis after severe ischemia-reperfusion or obstructive injury. Am. J. Physiol. Renal Physiol. 305, F477–F484 (2013).
Alikhan, M. A. et al. Colony-stimulating factor-1 promotes kidney growth and repair via alteration of macrophage responses. Am. J. Pathol. 179, 1243–1256 (2011).
Lee, S. et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol. 22, 317–326 (2011).
Anders, H. J. & Ryu, M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 80, 915–925 (2011).
Zhang, M. Z. et al. CSF-1 signaling mediates recovery from acute kidney injury. J. Clin. Invest. 122, 4519–4532 (2012).
Ranganathan, P. V., Jayakumar, C. & Ramesh, G. Netrin-1-treated macrophages protect the kidney against ischemia-reperfusion injury and suppress inflammation by inducing M2 polarization. Am. J. Physiol. Renal Physiol. 304, F948–F957 (2013).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Suzuki, N. et al. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature 416, 750–756 (2002).
Lech, M. et al. Macrophage phenotype controls long-term AKI outcomes—kidney regeneration versus atrophy. J. Am. Soc. Nephrol. 25, 292–304 (2014).
Penfield, J. G. et al. Transplant surgery injury recruits recipient MHC class II-positive leukocytes into the kidney. Kidney Int. 56, 1759–1769 (1999).
Dong, X. et al. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int. 71, 619–628 (2007).
Schlichting, C. L., Schareck, W. D. & Weis, M. Renal ischemia-reperfusion injury: new implications of dendritic cell-endothelial cell interactions. Transplant. Proc. 38, 670–673 (2006).
Loverre, A. et al. Ischemia-reperfusion injury-induced abnormal dendritic cell traffic in the transplanted kidney with delayed graft function. Kidney Int. 72, 994–1003 (2007).
Ozaki, K. S. et al. The loss of renal dendritic cells and activation of host adaptive immunity are long-term effects of ischemia/reperfusion injury following syngeneic kidney transplantation. Kidney Int. 81, 1015–1025 (2012).
Shau, H., Roth, M. D. & Golub, S. H. Regulation of natural killer function by nonlymphoid cells. Nat. Immun. 12, 235–249 (1993).
Chiche, L. et al. The role of natural killer cells in sepsis. J. Biomed. Biotechnol. 2011:986491 (2011).
Zhang, Z. X. et al. NK cells induce apoptosis in tubular epithelial cells and contribute to renal ischemia-reperfusion injury. J. Immunol. 181, 7489–7498 (2008).
Zhang, Z. X. et al. Osteopontin expressed in tubular epithelial cells regulates NK cell-mediated kidney ischemia reperfusion injury. J. Immunol. 185, 967–973 (2010).
Rabb, H. et al. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am. J. Physiol. Renal Physiol. 279, F525–F531 (2000).
Burne, M. J. et al. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J. Clin. Invest. 108, 1283–1290 (2001).
Ascon, D. B. et al. Phenotypic and functional characterization of kidney-infiltrating lymphocytes in renal ischemia reperfusion injury. J. Immunol. 177, 3380–3387 (2006).
Day, Y. J. et al. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-γ. J. Immunol. 176, 3108–3114 (2006).
Lai, L. W., Yong, K. C., Igarashi, S. & Lien, Y. H. A sphingosine-1-phosphate type 1 receptor agonist inhibits the early T-cell transient following renal ischemia-reperfusion injury. Kidney Int. 71, 1223–1231 (2007).
Sakr, M. et al. The protective effect of FK506 pretreatment against renal ischemia/reperfusion injury in rats. Transplantation 53, 987–991 (1992).
Jones, E. A. & Shoskes, D. A. The effect of mycophenolate mofetil and polyphenolic bioflavonoids on renal ischemia reperfusion injury and repair. J. Urol. 163, 999–1004 (2000).
Takada, M., Chandraker, A., Nadeau, K. C., Sayegh, M. H. & Tilney, N. L. The role of the B7 costimulatory pathway in experimental cold ischemia/reperfusion injury. J. Clin. Invest. 100, 1199–1203 (1997).
Chandraker, A. et al. CD28-b7 blockade in organ dysfunction secondary to cold ischemia/reperfusion injury. Kidney Int. 52, 1678–1684 (1997).
Yokota, N., Burne-Taney, M., Racusen, L. & Rabb, H. Contrasting roles for STAT4 and STAT6 signal transduction pathways in murine renal ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 285, F319–F325 (2003).
Wang, S. et al. Decreased renal ischemia-reperfusion injury by IL-16 inactivation. Kidney Int. 73, 318–326 (2008).
Gigliotti, J. C. et al. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J. Am. Soc. Nephrol. 24, 1451–1460 (2013).
Jang, H. R. et al. Early exposure to germs modifies kidney damage and inflammation after experimental ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 297, F1457–F1465 (2009).
Jang, H. R., Gandolfo, M. T., Ko, G. J., Racusen, L. & Rabb, H. The effect of murine anti-thymocyte globulin on experimental kidney warm ischemia-reperfusion injury in mice. Transpl. Immunol. 22, 44–54 (2009).
Satpute, S. R. et al. The role for T cell repertoire/antigen-specific interactions in experimental kidney ischemia reperfusion injury. J. Immunol. 183, 984–992 (2009).
Ko, G. J. et al. Blocking Fas ligand on leukocytes attenuates kidney ischemia-reperfusion injury. J. Am. Soc. Nephrol. 22, 732–742 (2011).
Ascon, M. et al. Renal ischemia-reperfusion leads to long term infiltration of activated and effector-memory T lymphocytes. Kidney Int. 75, 526–535 (2009).
Yokota, N., Daniels, F., Crosson, J. & Rabb, H. Protective effect of T cell depletion in murine renal ischemia-reperfusion injury. Transplantation 74, 759–763 (2002).
Park, P. et al. Injury in renal ischemia-reperfusion is independent from immunoglobulins and T lymphocytes. Am. J. Physiol. Renal Physiol. 282, F352–F357 (2002).
Gandolfo, M. T. et al. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 76, 717–729 (2009).
Gandolfo, M. T. et al. Mycophenolate mofetil modifies kidney tubular injury and Foxp3+ regulatory T cell trafficking during recovery from experimental ischemia-reperfusion. Transpl. Immunol. 23, 45–52 (2010).
Kinsey, G. R. et al. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J. Am. Soc. Nephrol. 20, 1744–1753 (2009).
Kinsey, G. R. et al. Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J. Am. Soc. Nephrol. 23, 1528–1537 (2012).
Kim, M. G. et al. CD4+ CD25+ regulatory T cells partially mediate the beneficial effects of FTY720, a sphingosine-1-phosphate analogue, during ischaemia/reperfusion-induced acute kidney injury. Nephrol. Dial. Transplant. 26, 111–124 (2011).
Kim, M. G. et al. IL-2/anti-IL-2 complex attenuates renal ischemia-reperfusion injury through expansion of regulatory T cells. J. Am. Soc. Nephrol. 24, 1529–1536 (2013).
Cho, W. Y. et al. The role of Tregs and CD11c(+) macrophages/dendritic cells in ischemic preconditioning of the kidney. Kidney Int. 78, 981–992 (2010).
Li, L. et al. NKT cell activation mediates neutrophil IFN-γ production and renal ischemia-reperfusion injury. J. Immunol. 178, 5899–5911 (2007).
Lee, H. T. et al. Isoflurane protects against renal ischemia and reperfusion injury and modulates leukocyte infiltration in mice. Am. J. Physiol. Renal Physiol. 293, F713–F722 (2007).
Yang, S. H. et al. Sulfatide-reactive natural killer T cells abrogate ischemia-reperfusion injury. J. Am. Soc. Nephrol. 22, 1305–1314 (2011).
Matsunaga, T. Did the first adaptive immunity evolve in the gut of ancient jawed fish? Cytogenet. Cell Genet. 80, 138–141 (1998).
Savransky, V. et al. Role of the T-cell receptor in kidney ischemia-reperfusion injury. Kidney Int. 69, 233–238 (2006).
Hochegger, K. et al. Role of α/β and γ/δ T cells in renal ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 293, F741–F747 (2007).
Burne-Taney, M. J. et al. B cell deficiency confers protection from renal ischemia reperfusion injury. J. Immunol. 171, 3210–3215 (2003).
Jang, H. R. et al. B cells limit repair after ischemic acute kidney injury. J. Am. Soc. Nephrol. 21, 654–665 (2010).
Fleming, S. D. et al. Mice deficient in complement receptors 1 and 2 lack a tissue injury-inducing subset of the natural antibody repertoire. J. Immunol. 169, 2126–2133 (2002).
Lobo, P. I. et al. Natural IgM anti-leukocyte autoantibodies attenuate excess inflammation mediated by innate and adaptive immune mechanisms involving Th-17. J. Immunol. 188, 1675–1685 (2012).
Faubel, S. et al. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1β, IL-18, IL-6, and neutrophil infiltration in the kidney. J. Pharmacol. Exp. Ther. 322, 8–15 (2007).
Zhang, B., Ramesh, G., Uematsu, S., Akira, S. & Reeves, W. B. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J. Am. Soc. Nephrol. 19, 923–932 (2008).
Yamate, J. et al. Immunohistochemical study of rat renal interstitial fibrosis induced by repeated injection of cisplatin, with special reference to the kinetics of macrophages and myofibroblasts. Toxicol. Pathol. 24, 199–206 (1996).
Lu, L. H. et al. Increased macrophage infiltration and fractalkine expression in cisplatin-induced acute renal failure in mice. J. Pharmacol. Exp. Ther. 324, 111–117 (2008).
Tadagavadi, R. K. & Reeves, W. B. Renal dendritic cells ameliorate nephrotoxic acute kidney injury. J. Am. Soc. Nephrol. 21, 53–63 (2010).
Tadagavadi, R. K. & Reeves, W. B. Endogenous IL-10 attenuates cisplatin nephrotoxicity: role of dendritic cells. J. Immunol. 185, 4904–4911 (2010).
Liu, M. et al. A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity. J. Am. Soc. Nephrol. 17, 765–774 (2006).
Linkermann, A. et al. Renal tubular Fas ligand mediates fratricide in cisplatin-induced acute kidney failure. Kidney Int. 79, 169–178 (2011).
Lee, H. et al. CD4+CD25+ regulatory T cells attenuate cisplatin-induced nephrotoxicity in mice. Kidney Int 78, 1100–1109 (2010).
Eller, K. et al. CCR7 deficiency exacerbates injury in acute nephritis due to aberrant localization of regulatory T cells. J. Am. Soc. Nephrol. 21, 42–52 (2010).
Acknowledgements
H.R.J. is supported by a grant from the Korean Health Technology Research & Development Project, Ministry of Health & Welfare, Republic of Korea (HI13C-1263-020,013), a grant from Samsung Biomedical Research Institute (GL1B22612), and a grant from the Korean Society of Nephrology (PHX1130221). H.R. is supported by the NIH, US National Kidney Foundation, and generous research support from Rogelio Miro, Anne Segerson and Stoney Stanfil.
Author information
Authors and Affiliations
Contributions
H.R.J. wrote the manuscript and researched data for the article; H.R.J. and H.R. contributed equally to discussion of content, review and/or editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Jang, H., Rabb, H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol 11, 88–101 (2015). https://doi.org/10.1038/nrneph.2014.180
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2014.180
This article is cited by
-
Sulforaphane (Sul) reduces renal interstitial fibrosis (RIF) by controlling the inflammation and TGF-β/Smad signaling pathway
Applied Biological Chemistry (2024)
-
Effects of poly (ADP-ribose) polymerase inhibitor treatment on the repair process of ischemic acute kidney injury
Scientific Reports (2024)
-
Identification of TACSTD2 as novel therapeutic targets for cisplatin-induced acute kidney injury by multi-omics data integration
Human Genetics (2024)
-
Empagliflozin improves renal ischemia–reperfusion injury by reducing inflammation and enhancing mitochondrial fusion through AMPK–OPA1 pathway promotion
Cellular & Molecular Biology Letters (2023)
-
High-dose atorvastatin reduces oxidative stress of ischemia/reperfusion injury after isogeneic kidney transplantation in rats: in vivo, preclinical, case–control, open-label study
Renal Replacement Therapy (2023)