Key Points
-
Renal involvement in primary Sjögren syndrome (pSS) is a rare complication that occurs in <10% patients and usually has a favourable prognosis
-
Tubulointerstitial nephritis (TIN) is the most frequent renal complication of pSS, and is characterized by infiltration of the interstitium with plasma cells and lymphocytes
-
Patients with pSS and TIN usually respond to steroid therapy alone; immunosuppressive agents and/or B-cell targeted therapies might enable steroid sparing but their use remains to be established
-
Patients with pSS, cryoglobulinaemia and low serum complement levels have a high risk of developing lymphoma and/or membranoproliferative glomerulonephritis, which responds well to steroid treatment plus rituximab and plasma exchange
-
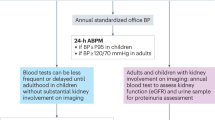
Renal disease in pSS is often pauci-symptomatic; appropriate biologic screening of the serum and urine is required to detect and prevent terminal chronic kidney disease
-
Identification of renal disease in suspected pSS should prompt an investigation to exclude other inflammatory disorders, such as lupus erythematosus, hepatitis C virus infection and IgG4-associated disorders
Abstract
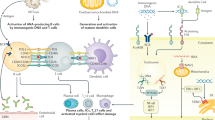
Primary Sjögren syndrome (pSS) is an autoimmune disorder characterized by lymphoplasmacytic infiltration of the exocrine (salivary and lachrymal) glands that results in sicca symptoms (dryness of the eyes and mouth). Systemic complications can occur in pSS, but renal involvement is rare, affecting <10% patients. The most frequent form of nephropathy in pSS is tubulointerstitial nephritis (TIN), in which infiltration of the kidney by plasma cells is a key feature and shows similarity to the lymphoplasmacytic infiltration of the salivary glands. Electrolyte disturbances may occur in pSS, such as renal distal tubular acidosis, diabetes insipidus, Gitelman syndrome or Fanconi syndrome. Glomerular involvement is less frequently detected in patients with pSS, but usually takes the form of membranoproliferative glomerulonephritis secondary to cryoglobulinaemia. The renal prognosis in patients with pSS and TIN or glomerular disease is usually favourable, but the risk of chronic kidney disease remains high in patients with TIN. Appropriate screening must be performed at least once a year in patients with systemic pSS in order to facilitate the early detection of renal complications. In this Review we discuss the epidemiology, pathophysiology, differential diagnosis and treatment of renal disease in pSS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ramos-Casals, M., Brito-Zerón, P., Sisó-Almirall, A. & Bosch, X. Primary Sjögren syndrome. BMJ 344, e3821 (2012).
Qin, B. et al. Epidemiology of primary Sjögren's syndrome: a systematic review and meta-analysis. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2014-205375.
Bossini, N. et al. Clinical and morphological features of kidney involvement in primary Sjögren's syndrome. Nephrol. Dial. Transplant. 16, 2328–2336 (2001).
Thieblemont, C., Berger, F. & Coiffier, B. Mucosa-associated lymphoid tissue lymphomas. Curr. Opin. Oncol. 7, 415–420 (1995).
Tzioufas, A. G. & Voulgarelis, M. Update on Sjögren's syndrome autoimmune epithelitis: from classification to increased neoplasias. Best Pract. Res. Clin. Rheumatol. 21, 989–1010 (2007).
Malladi, A. S. et al. Primary Sjögren's syndrome as a systemic disease: a study of participants enrolled in an international Sjögren's syndrome registry. Arthritis Care Res. 64, 911–918 (2012).
Baldini, C. et al. Primary Sjögren's syndrome as a multi-organ disease: impact of the serological profile on the clinical presentation of the disease in a large cohort of Italian patients. Rheumatology (Oxford) 53, 839–844 (2014).
Lin, D.-F. et al. Clinical and prognostic characteristics of 573 cases of primary Sjögren's syndrome. Chin. Med. J. (Engl.) 123, 3252–3257 (2010).
Vitali, C. et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann. Rheum. Dis. 61, 554–558 (2002).
Vitali, C. et al. Preliminary criteria for the classification of Sjögren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum. 36, 340–347 (1993).
Vitali, C. et al. Assessment of the European classification criteria for Sjögren's syndrome in a series of clinically defined cases: results of a prospective multicentre study. The European Study Group on Diagnostic Criteria for Sjögren's Syndrome. Ann. Rheum. Dis. 55, 116–121 (1996).
Shiboski, S. C. et al. American College of Rheumatology classification criteria for Sjögren's syndrome: a data-driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance cohort. Arthritis Care Res. (Hoboken) 64, 475–487 (2012).
Seror, R. et al. EULAR Sjögren's syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjögren's syndrome. Ann. Rheum. Dis. 69, 1103–1109 (2010).
Gottenberg, J.-E. et al. Serum levels of β2-microglobulin and free light chains of immunoglobulins are associated with systemic disease activity in primary Sjögren's syndrome. Data at enrollment in the prospective ASSESS cohort. PLoS ONE 8, e59868 (2013).
Iwakiri, D. et al. Epstein–Barr virus (EBV)-encoded small RNA is released from EBV-infected cells and activates signaling fromToll-like receptor 3. J. Exp. Med. 206, 2091–2099 (2009).
Nocturne, G. & Mariette, X. Advances in understanding the pathogenesis of primary Sjögren's syndrome. Nat. Rev. Rheumatol. 9, 544–556 (2013).
Tsunawaki, S. et al. Possible function of salivary gland epithelial cells as nonprofessional antigen-presenting cells in the development of Sjögren's syndrome. J. Rheumatol. 29, 1884–1896 (2002).
Ittah, M. et al. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjögren's syndrome. Arthritis Res. Ther. 8, R51 (2006).
Fukatsu, A. et al. Expression of interleukin 6 and major histocompatibility complex molecules in tubular epithelial cells of diseased human kidneys. Lab. Invest. 69, 58–67 (1993).
Oren, R. et al. C3, C4, factor B and HLA-DR α mRNA expression in renal biopsy specimens from patients with IgA nephropathy. Immunology 86, 575–583 (1995).
Maripuri, S. et al. Renal involvement in primary Sjögren's syndrome: a clinicopathologic study. Clin. J. Am. Soc. Nephrol. 4, 1423–1431 (2009).
Ren, H. et al. Renal involvement and follow-up of 130 patients with primary Sjögren's syndrome. J. Rheumatol. 35, 278–284 (2008).
Talal, N., Zisman, E. & Schur, P. H. Renal tubular acidosis, glomerulonephritis and immunologic factors in Sjögren's syndrome. Arthritis Rheum. 11, 774–786 (1968).
Shearn, M. A. & Tu, W. H. Nephrogenic diabetic insipidus and other defects of renal tubular function in sjoergren's syndrome. Am. J. Med. 39, 312–318 (1965).
Goules, A. V., Tatouli, I. P., Moutsopoulos, H. M. & Tzioufas, A. G. Clinically significant renal involvement in primary Sjögren's syndrome: clinical presentation and outcome. Arthritis Rheum. 65, 2945–2953 (2013).
Cimaz, R. et al. Primary Sjögren syndrome in the paediatric age: a multicentre survey. Eur. J. Pediatr. 162, 661–665 (2003).
De Souza, T. R. et al. Juvenile Sjögren syndrome: distinctive age, unique findings. Pediatr. Dent. 34, 427–430 (2012).
Goules, A. et al. Clinically significant and biopsy-documented renal involvement in primary Sjögren syndrome. Medicine (Baltimore) 79, 241–249 (2000).
Bridoux, F. et al. Proximal tubular dysfunction in primary Sjögren's syndrome: a clinicopathological study of 2 cases. Clin. Nephrol. 61, 434–439 (2004).
Kim, Y. K. et al. Acquired Gitelman syndrome in a patient with primary Sjögren syndrome. Am. J. Kidney Dis. 52, 1163–1167 (2008).
Cohen, E. P. et al. Absence of H+-ATPase in cortical collecting tubules of a patient with Sjögren's syndrome and distal renal tubular acidosis. J. Am. Soc. Nephrol. 3, 264–271 (1992).
DeFranco, P. E., Haragsim, L., Schmitz, P. G. & Bastani, B. Absence of vacuolar H+-ATPase pump in the collecting duct of a patient with hypokalemic distal renal tubular acidosis and Sjögren's syndrome. J. Am. Soc. Nephrol. 6, 295–301 (1995).
Inagaki, Y., Jinno-Yoshida, Y., Hamasaki, Y. & Ueki, H. A novel autoantibody reactive with carbonic anhydrase in sera from patients with systemic lupus erythematosus and Sjögren's syndrome. J. Dermatol. Sci. 2, 147–154 (1991).
Pertovaara, M., Bootorabi, F., Kuuslahti, M., Pasternack, A. & Parkkila, S. Novel carbonic anhydrase autoantibodies and renal manifestations in patients with primary Sjögren's syndrome. Rheumatology (Oxford) 50, 1453–1457 (2011).
Takemoto, F. et al. Induction of anti-carbonic-anhydrase-II antibody causes renal tubular acidosis in a mouse model of Sjögren's syndrome. Nephron Physiol. 106, 63–68 (2007).
Kim, J., Tisher, C. C., Linser, P. J. & Madsen, K. M. Ultrastructural localization of carbonic anhydrase II in subpopulations of intercalated cells of the rat kidney. J. Am. Soc. Nephrol. 1, 245–256 (1990).
Pertovaara, M., Korpela, M., Kouri, T. & Pasternack, A. The occurrence of renal involvement in primary Sjögren's syndrome: a study of 78 patients. Rheumatology (Oxford) 38, 1113–1120 (1999).
Aasarød, K., Haga, H. J., Berg, K. J., Hammerstrøm, J. & Jørstad, S. Renal involvement in primary Sjögren's syndrome. QJM 93, 297–304 (2000).
Saeki, T. & Kawano, M. IgG4-related kidney disease. Kidney Int. 85, 251–257 (2014).
Mavragani, C. P., Fragoulis, G. E., Rontogianni, D., Kanariou, M. & Moutsopoulos, H. M. Elevated IgG4 serum levels among primary Sjögren's syndrome patients: do they unmask underlying IgG4-related disease? Arthritis Care Res. 66, 773–777 (2014).
Kawano, M. et al. Primary Sjögren's syndrome with chronic tubulointerstitial nephritis and lymphadenopathy mimicking IgG4-related disease. Mod. Rheumatol. 25, 637–641 (2013).
Nakashima, Y. et al. Comorbid case of IgG4-related disease and primary Sjögren's syndrome. Mod. Rheumatol. 25, 462–467 (2015).
Johnson, R. J., Feehally, J. & Floege, J. (eds). Comprehensive Clinical Nephrology 5th edn (Saunders, 2014).
Akiyama, Y. et al. A case of sarcoidosis associated with Sjögren's syndrome [Japanese]. Arerugi 41, 1500–1506 (1992).
Sharma, S., Gupta, A. & Saxena, S. Comprehensive clinical approach to renal tubular acidosis. Clin. Exp. Nephrol. 19, 556–561 (2015).
Haque, S. K., Ariceta, G. & Batlle, D. Proximal renal tubular acidosis: a not so rare disorder of multiple etiologies. Nephrol. Dial. Transplant. 27, 4273–4287 (2012).
Fremont, O. T. & Chan, J. C. Understanding Bartter syndrome and Gitelman syndrome. World J. Pediatr. 8, 25–30 (2012).
Hinschberger, O. et al. Acquired Gitelman syndrome associated with Sjögren's syndrome and scleroderma [French]. Rev. Méd. Interne. 32, e96–e98 (2011).
Casatta, L., Ferraccioli, G. F. & Bartoli, E. Hypokalaemic alkalosis, acquired Gitelman's and Bartter's syndrome in chronic sialoadenitis. Br. J. Rheumatol. 36, 1125–1128 (1997).
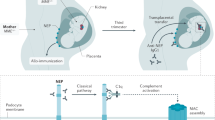
Matignon, M. et al. Clinical and morphologic spectrum of renal involvement in patients with mixed cryoglobulinemia without evidence of hepatitis C virus infection. Medicine (Baltimore) 88, 341–348 (2009).
Terrier, B. et al. Management of noninfectious mixed cryoglobulinemia vasculitis: data from 242 cases included in the CryoVas survey. Blood 119, 5996–6004 (2012).
D'Amico, G. & Fornasieri, A. Cryoglobulinemic glomerulonephritis: a membranoproliferative glomerulonephritis induced by hepatitis C virus. Am. J. Kidney Dis. 25, 361–369 (1995).
Cacoub, P., Terrier, B. & Saadoun, D. Hepatitis C virus-induced vasculitis: therapeutic options. Ann. Rheum. Dis. 73, 24–30 (2014).
Mekinian, A. et al. Efficacy of rituximab in primary Sjögren's syndrome with peripheral nervous system involvement: results from the AIR registry. Ann. Rheum. Dis. 71, 84–87 (2012).
Terrier, B. et al. Safety and efficacy of rituximab in nonviral cryoglobulinemia vasculitis: data from the French Autoimmunity and Rituximab registry. Arthritis Care Res. 62, 1787–1795 (2010).
Gottenberg, J.-E. et al. Efficacy of rituximab in systemic manifestations of primary Sjögren's syndrome: results in 78 patients of the AutoImmune and Rituximab registry. Ann. Rheum. Dis. 72, 1026–1031 (2013).
Sethi, S. & Fervenza, F. C. Membranoproliferative glomerulonephritis—a new look at an old entity. N. Engl. J. Med. 366, 1119–1131 (2012).
Leadbetter, E. A. et al. Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416, 603–607 (2002).
Hsieh, C. et al. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res. 63, 865–874 (2011).
Alsuwaida, A. O. Interstitial inflammation and long-term renal outcomes in lupus nephritis. Lupus 22, 1446–1454 (2013).
Baba, A. et al. Three patients with nephrotic syndrome due to membranous nephropathy complicated by Sjögren's syndrome [Japanese]. Nihon Jinzo Gakkai Shi 47, 882–886 (2005).
Font, J., Cervera, R., Lopez-Soto, A., Darnell, A. & Ingelmo, M. Mixed membranous and proliferative glomerulonephritis in primary Sjögren's syndrome. Br. J. Rheumatol. 28, 548–550 (1989).
Siamopoulos, K. C., Mavridis, A. K., Elisaf, M., Drosos, A. A. & Moutsopoulos, H. M. Kidney involvement in primary Sjögren's syndrome. Scand. J. Rheumatol. Suppl. 61, 156–160 (1986).
Ronco, P. & Debiec, H. Pathogenesis of membranous nephropathy: recent advances and future challenges. Nat. Rev. Nephrol. 8, 203–213 (2012).
Yang, M.-L., Kuo, M.-C., Ou, T.-T. & Chen, H.-C. Primary Sjögren's syndrome with minimal change disease—a case report. Kaohsiung J. Med. Sci. 27, 190–194 (2011).
Dussol, B. et al. Crescentic glomerulonephritis and primary Gougerot-Sjögren syndrome [French]. Nephrologie 15, 295–298 (1994).
Kamachi, M. et al. Sjögren's syndrome complicated by MPO-ANCA positive crescentic glomerulonephritis. Nephrol. Dial. Transplant. 14, 1033–1034 (1999).
Tatsumi, H., Tateno, S., Hiki, Y. & Kobayashi, Y. Crescentic glomerulonephritis and primary Sjögren's syndrome. Nephron 86, 505–506 (2000).
Wang, W.-J., Wu, H.-S. & Chu, T.-S. Anti-neutrophil cytoplasmic antibody-associated Pauci-immune crescentic glomerulonephritis complicating Sjögren's syndrome. J. Formos. Med. Assoc. 110, 473–477 (2011).
Guellec, D. et al. ANCA-associated vasculitis in patients with primary Sjögren's syndrome: detailed analysis of 7 new cases and systematic literature review. Autoimmun. Rev. 14, 742–750 (2015).
Skopouli, F. N., Dafni, U., Ioannidis, J. P. & Moutsopoulos, H. M. Clinical evolution, and morbidity and mortality of primary Sjögren's syndrome. Semin. Arthritis Rheum. 29, 296–304 (2000).
García-Carrasco, M. et al. Primary Sjögren syndrome: clinical and immunologic disease patterns in a cohort of 400 patients. Medicine (Baltimore) 81, 270–280 (2002).
Ramos-Casals, M. et al. Primary Sjögren syndrome in Spain: clinical and immunologic expression in 1010 patients. Medicine (Baltimore) 87, 210–219 (2008).
Ramos-Casals, M. et al. Systemic involvement in primary Sjögren's syndrome evaluated by the EULAR-SS disease activity index: analysis of 921 Spanish patients (GEAS-SS Registry). Rheumatology (Oxford) 53, 321–331 (2014).
Acknowledgements
The authors would like to thank Dr Charlotte Mussini (Department of Pathology, Bicetre Hospital, Assistance Publique-Hôpitaux de Paris) for providing the histologic images and Dr Magali Jasiek (Nephrology Department, Bicetre Hospital, Assistance Publique-Hôpitaux de Paris) for her contribution to Tables 2 and 3.
Author information
Authors and Affiliations
Contributions
H.F. and X.M. researched the data for the article, provided a substantial contribution to discussions of the content, contributed equally to writing the article and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
François, H., Mariette, X. Renal involvement in primary Sjögren syndrome. Nat Rev Nephrol 12, 82–93 (2016). https://doi.org/10.1038/nrneph.2015.174
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2015.174
This article is cited by
-
Kidney manifestations of pediatric Sjögren’s syndrome
Pediatric Nephrology (2024)
-
Renal tubular acidosis without interstitial nephritis in Sjögren’s syndrome: a case report and review of the literature
BMC Nephrology (2023)
-
Related factors of renal injury in primary Sjögren's syndrome
Immunity & Ageing (2023)
-
Childhood-onset primary Sjögren’s syndrome in a tertiary center in China: clinical features and outcome
Pediatric Rheumatology (2023)
-
The pathophysiology of distal renal tubular acidosis
Nature Reviews Nephrology (2023)