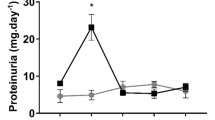
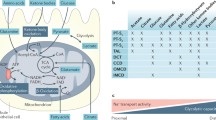
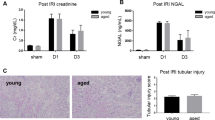
Key Points
-
Acute injury to the kidney is often associated with maladaptive repair and incomplete resolution, leading to residual abnormalities in kidney structure and function
-
Increasing age, and chronic low-grade insults to the tubular epithelium increase epithelial cell sensitivity to episodes of acute kidney injury, leading to maladaptive repair and progression of chronic kidney disease
-
Maladaptive repair is characterized by fibrosis, vascular rarefaction, tubular loss, glomerulosclerosis and the presence of a chronic inflammatory infiltrate within the kidney
-
Injured renal tubular epithelial cells become arrested at G2/M and adopt a profibrotic phenotype, which affects other epithelial cells, pericytes and the immune system
-
Myofibroblasts that likely arise from renal pericytes proliferate and contribute to the deposition of extracellular matrix and resulting fibrosis within the injured kidney
-
Maladaptive repair after acute kidney insults shares many common features with kidney ageing and can be thought of as a state of accelerated kidney ageing
Abstract
Acute kidney injury is an increasingly common complication of hospital admission and is associated with high levels of morbidity and mortality. A hypotensive, septic, or toxic insult can initiate a cascade of events, resulting in impaired microcirculation, activation of inflammatory pathways and tubular cell injury or death. These processes ultimately result in acutely impaired kidney function and initiation of a repair response. This Review explores the various mechanisms responsible for the initiation and propagation of acute kidney injury, the prototypic mechanisms by which a substantially damaged kidney can regenerate its normal architecture, and how the adaptive processes of repair can become maladaptive. These mechanisms, which include G2/M cell-cycle arrest, cell senescence, profibrogenic cytokine production, and activation of pericytes and interstitial myofibroblasts, contribute to the development of progressive fibrotic kidney disease. The end result is a state that mimics accelerated kidney ageing. These mechanisms present important opportunities for the design of targeted therapeutic strategies to promote adaptive renal recovery and minimize progressive fibrosis and chronic kidney disease after acute insults.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Metcalfe, W. et al. Acute renal failure requiring renal replacement therapy: incidence and outcome. QJM 95, 579–583 (2002).
Ali, T. et al. Incidence and outcomes in acute kidney injury: A comprehensive population-based study. J. Am. Soc. Nephrol. 18, 1292–1298 (2007).
Noble, J. S., Simpson, K. & Allison, M. E. Long-term quality of life and hospital mortality in patients treated with intermittent or continuous hemodialysis for acute renal and respiratory failure. Ren. Fail. 28, 323–330 (2006).
Xue, J. L. et al. Incidence and mortality of acute renal failure in medicare beneficiaries, 1992 to 2001. J. Am. Soc. Nephrol. 17, 1135–1142 (2006).
Wonnacott, A., Meran, S., Amphlett, B., Talabani, B. & Phillips, A. Epidemiology and outcomes in community-acquired versus hospital-acquired aki. Clin. J. Am. Soc. Nephrol. 9, 1007–1014 (2014).
Thadhani, R., Pascual, M. & Bonventre, J. V. Acute renal failure. N. Engl. J. Med. 334, 1448–1460 (1996).
Brezis, M. & Rosen, S. Hypoxia of the renal medulla—its implications for disease. N. Engl. J. Med. 332, 647–655 (1995).
Friedrich, J. O., Adhikari, N., Herridge, M. S. & Beyene, J. Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann. Intern. Med. 142, 510–524 (2005).
Ho, K. M. & Sheridan, D. J. Meta-analysis of frusemide to prevent or treat acute renal failure. BMJ 333, 420 (2006).
Coca, S. G., Singanamala, S. & Parikh, C. R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 81, 442–448 (2012).
Ishani, A. et al. Acute kidney injury increases risk of ESRD among elderly. J. Am. Soc. Nephrol. 20, 223–228 (2009).
Shimoi, T. et al. The significant impact of acute kidney injury on CKD in patients who survived over 10 years after myeloablative allogeneic SCT. Bone Marrow Transplant. 48, 80–84 (2013).
Belayev, L. Y. & Palevsky, P. M. The link between acute kidney injury and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. (2013).
Chawla, L. S. & Kimmel, P. L. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 82, 516–524 (2012).
Yang, L., Besschetnova, T. Y., Brooks, C. R., Shah, J. V. & Bonventre, J. V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 16, 535–543 (2010).
Grgic, I. et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 82, 172–183 (2012).
Basile, D. P., Donohoe, D., Roethe, K. & Osborn, J. L. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am. J. Physiol. Renal Physiol. 281, F887–F899 (2001).
Schmitt, R. & Cantley, L. G. The impact of aging on kidney repair. Am. J. Physiol. Renal Physiol. 294, F1265–F1272 (2008).
Bonventre, J. V. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J. Am. Soc. Nephrol. 14 (Suppl. 1), S55–S61 (2003).
Cantley, L. G. Adult stem cells in the repair of the injured renal tubule. Nat. Clin. Pract. Nephrol. 1, 22–32 (2005).
Oliver, J. A., Maarouf, O., Cheema, F. H., Martens, T. P. & Al-Awqati, Q. The renal papilla is a niche for adult kidney stem cells. J. Clin. Invest. 114, 795–804 (2004).
Bussolati, B, et al. Isolation of renal progenitor cells from adult human kidney. Am. J. Pathol. 166, 545–555 (2005).
Dekel, B. et al. Isolation and characterization of nontubular sca-1+lin− multipotent stem/progenitor cells from adult mouse kidney. J. Am. Soc. Nephrol. 17, 3300–3314 (2006).
Humphreys, B. D. et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2, 284–291 (2008).
Humphreys, B. D. et al. Repair of injured proximal tubule does not involve specialized progenitors. Proc. Natl Acad. Sci. USA 108, 9226–9231 (2011).
Berger, K. & Moeller, M. J. Mechanisms of epithelial repair and regeneration after acute kidney injury. Semin. Nephrol. 34, 394–403 (2014).
Evans, R. G. et al. Haemodynamic influences on kidney oxygenation: clinical implications of integrative physiology. Clin. Exp. Pharmacol. Physiol. 40, 106–122 (2013).
Pallone, T. L., Robertson, C. R. & Jamison, R. L. Renal medullary microcirculation. Physiol. Rev. 70, 885–920 (1990).
Basile, D. P. The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int. 72, 151–156 (2007).
Kim, M. et al. The volatile anesthetic isoflurane induces ecto-5'-nucleotidase (cd73) to protect against renal ischemia and reperfusion injury. Kidney Int. 84, 90–103 (2013).
Venkatachalam, M. A. & Weinberg, J. M. The conundrum of protection from AKI by adenosine in rodent clamp ischemia models. Kidney Int. 84, 16–19 (2013).
Conger, J. D., Robinette, J. B. & Hammond, W. S. Differences in vascular reactivity in models of ischemic acute renal failure. Kidney Int. 39, 1087–1097 (1991).
Noiri, E. et al. Oxidative and nitrosative stress in acute renal ischemia. Am. J. Physiol. Renal Physiol. 281, F948–F957 (2001).
De Greef, K. E., Ysebaert, D. K., Persy, V., Vercauteren, S. R. & De Broe, M. E. ICAM-1 expression and leukocyte accumulation in inner stripe of outer medulla in early phase of ischemic compared to hgCl2-induced ARF. Kidney Int. 63, 1697–1707 (2003).
Kelly, K. J. et al. Minocycline inhibits apoptosis and inflammation in a rat model of ischemic renal injury. Am. J. Physiol. Renal Physiol. 287, F760–F766 (2004).
Brodsky, S. V. et al. Endothelial dysfunction in ischemic acute renal failure: rescue by transplanted endothelial cells. Am. J. Physiol. Renal Physiol. 282, F1140–F1149 (2002).
Yamamoto, T. et al. Intravital videomicroscopy of peritubular capillaries in renal ischemia. Am. J. Physiol. Renal Physiol. 282, F1150–F1155 (2002).
Schrimpf, C. & Duffield, J. S. Mechanisms of fibrosis: the role of the pericyte. Curr. Opin. Nephrol. Hypertens. 20, 297–305 (2011).
Humphreys, B. D. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 176, 85–97 (2010).
Schrimpf, C. et al. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J. Am. Soc. Nephrol. 23, 868–883 (2012).
Lee, S. et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol. 22, 317–326 (2011).
Dong, X. et al. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int. 71, 619–628 (2007).
Ysebaert, D. K. et al. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol. Dial. Transplant. 15, 1562–1574 (2000).
Kelly, K. J. et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J. Clin. Invest. 97, 1056–1063 (1996).
Bolisetty, S. & Agarwal, A. Neutrophils in acute kidney injury: not neutral any more. Kidney Int. 75, 674–676 (2009).
Li, L. et al. IL-17 produced by neutrophils regulates IFN-γ-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J. Clin. Invest. 120, 331–342 (2010).
Chaturvedi, S. et al. Slit2 prevents neutrophil recruitment and renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 24, 1274–1287 (2013).
Awad, A. S. et al. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int. 75, 689–698 (2009).
Burne-Taney, M. J., Yokota-Ikeda, N. & Rabb, H. Effects of combined T- and B-cell deficiency on murine ischemia reperfusion injury. Am. J. Transplant. 5, 1186–1193 (2005).
Melnikov, V. Y. et al. Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J. Clin. Invest. 110, 1083–1091 (2002).
Thornton, M. A., Winn, R., Alpers, C. E. & Zager, R. A. An evaluation of the neutrophil as a mediator of in vivo renal ischemic-reperfusion injury. Am. J. Pathol. 135, 509–515 (1989).
Savill, J., Smith, J., Sarraf, C., Ren, Y., Abbott, F. & Rees, A. Glomerular mesangial cells and inflammatory macrophages ingest neutrophils undergoing apoptosis. Kidney Int. 42, 924–936 (1992).
Ferenbach, D. A. et al. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int. 82, 928–933 (2012).
Jo, S. K., Sung, S. A., Cho, W. Y., Go, K. J. & Kim, H. K. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol. Dial. Transplant. 21, 1231–1239 (2006).
Day, Y. J., Huang, L., Ye, H., Linden, J. & Okusa, M. D. Renal ischemia-reperfusion injury and adenosine 2a receptor-mediated tissue protection: Role of macrophages. Am. J. Physiol. Renal Physiol. 288, F722–F731 (2005).
Rae, F. et al. Characterisation and trophic functions of murine embryonic macrophages based upon the use of a csf1r-egfp transgene reporter. Dev. Biol. 308, 232–246 (2007).
Ricardo S. D., van Goor, H. & Eddy, A. A. Macrophage diversity in renal injury and repair. J. Clin. Invest. 118, 3522–3530 (2008).
Lin, S. L. et al. Macrophage wnt7b is critical for kidney repair and regeneration. Proc. Natl Acad. Sci. USA 107, 4194–4199 (2010).
Jang, H. S., Kim, J., Park, Y. K. & Park, K. M. Infiltrated macrophages contribute to recovery after ischemic injury but not to ischemic preconditioning in kidneys. Transplantation 85, 447–455 (2008).
Lech, M. et al. Macrophage phenotype controls long-term AKI outcomes—kidney regeneration versus atrophy. J. Am. Soc. Nephrol. 25, 292–304 (2013).
Linfert, D., Chowdhry, T. & Rabb, H. Lymphocytes and ischemia-reperfusion injury. Transplant. Rev. 23, 1–10 (2009).
Burne-Taney, M. J. et al. B cell deficiency confers protection from renal ischemia reperfusion injury. J. Immunol. 171, 3210–3215 (2003).
Rabb, H. et al. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am. J. Physiol. Renal Physiol. 27, F525–F531 (2000).
Park, P. et al. Injury in renal ischemia–reperfusion is independent from immunoglobulins and T lymphocytes. Am. J. Physiol. Renal Physiol. 282, F352–F357 (2002).
Kinsey, G. R. et al. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J. Am. Soc. Nephrol. 20, 1744–1753 (2009).
Jang, H. R. et al. B cells limit repair after ischemic acute kidney injury. J. Am. Soc. Nephrol. 21, 654–665 (2010).
Burne-Taney, M. J. et al. Transfer of lymphocytes from mice with renal ischemia can induce albuminuria in naive mice: A possible mechanism linking early injury and progressive renal disease? Am. J. Physiol. Renal Physiol. 291, F981–F986 (2006).
Day, Y. J. et al. Renal protection from ischemia mediated by a2a adenosine receptors on bone marrow-derived cells. J. Clin. Invest. 112, 883–891 (2003).
Basile, D. P. et al. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am. J. Physiol. Renal Physiol. 300, F721–F733 (2011).
Li, L. et al. Dendritic cells tolerized with adenosine A2 AR agonist attenuate acute kidney injury. J. Clin. Invest. 122, 3931–3942 (2012).
Kinsey, G. R. et al. Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J. Am. Soc. Nephrol. 23, 1528–1537 (2012).
Ferenbach, D. A. et al. The induction of macrophage hemeoxygenase-1 is protective during acute kidney injury in aging mice. Kidney Int. 79, 966–976 (2011).
Ferenbach, D. A. et al. Macrophages expressing heme oxygenase-1 improve renal function in ischemia/reperfusion injury. Mol. Ther. 18, 1706–1713 (2010).
Duffield, J. S. et al. Resolvin D series and protectin D1 mitigate acute kidney injury. J. Immunol. 177, 5902–5911 (2006).
Leonard, M. O. et al. 15-epi-16-(para-fluorophenoxy)-lipoxin a(4)-methyl ester, a synthetic analogue of 15-epi-lipoxin A(4), is protective in experimental ischemic acute renal failure. J. Am. Soc. Nephrol. 13, 1657–1662 (2002).
Franquesa, M. et al. Tubular epithelial cells transfected with hHGF counteracts monocyte chemotactic protein-1 up-regulation after hypoxia/reoxygenation insult. Transplant. Proc. 41, 2069–2072 (2009).
Franquesa, M. et al. Direct electrotransfer of hHGF gene into kidney ameliorates ischemic acute renal failure. Gene Ther. 12, 1551–1558 (2005).
Thurman, J. M., Lucia, M. S., Ljubanovic, D. & Holers, V. M. Acute tubular necrosis is characterized by activation of the alternative pathway of complement. Kidney Int. 67, 524–530 (2005).
Thurman, J. M. et al. Altered renal tubular expression of the complement inhibitor Crry permits complement activation after ischemia/reperfusion. J. Clin. Invest. 116, 357–368 (2006).
Peng, Q., Li, K., Patel, H., Sacks, S. H. & Zhou, W. Dendritic cell synthesis of C3 is required for full T cell activation and development of a th1 phenotype. J. Immunol. 176, 3330–3341 (2006).
Thurman, J. M. et al. Treatment with an inhibitory monoclonal antibody to mouse factor b protects mice from induction of apoptosis and renal ischemia/reperfusion injury. J. Am. Soc. Nephrol. 17, 707–715 (2006).
Renner, B. et al. The complement inhibitors Crry and factor H are critical for preventing autologous complement activation on renal tubular epithelial cells. J. Immunol. 185, 3086–3094 (2010).
Linkermann, A. et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc. Natl Acad. Sci. USA 110, 12024–12029 (2013).
Linkermann, A., De Zen, F., Weinberg, J., Kunzendorf, U. & Krautwald, S. Programmed necrosis in acute kidney injury. Nephrol. Dial. Transplant. 27, 3412–3419 (2012).
Ishibe, S. & Cantley, L. G. Epithelial–mesenchymal–epithelial cycling in kidney repair. Curr. Opin. Nephrol. Hypertens. 17, 379–385 (2008).
Villanueva, S., Cespedes, C. & Vio, C. P. Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R861–R870 (2006).
Witzgall, R., Brown, D., Schwarz, C. & Bonventre, J. V. Localization of proliferating cell nuclear antigen, vimentin, c-fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J. Clin. Invest. 93, 2175–2188 (1994).
Witzgall, R., O'Leary, E., Gessner, R., Ouellette, A. J. & Bonventre, J. V. Kid-1, a putative renal transcription factor: Regulation during ontogeny and in response to ischemia and toxic injury. Mol. Cell. Biol. 13, 1933–1942 (1993).
Price, V. R., Reed, C. A., Lieberthal, W. & Schwartz, J. H. ATP depletion of tubular cells causes dissociation of the zonula adherens and nuclear translocation of β-catenin and LEF-1. J. Am. Soc. Nephrol. 13, 1152–1161 (2002).
Kuure, S., Popsueva, A., Jakobson, M., Sainio, K. & Sariola, H. Glycogen synthase kinase-3 inactivation and stabilization of β-catenin induce nephron differentiation in isolated mouse and rat kidney mesenchymes. J. Am. Soc. Nephrol. 18, 1130–1139 (2007).
Ishibe, S., Haydu, J. E., Togawa, A., Marlier, A. & Cantley, L. G. Cell confluence regulates hepatocyte growth factor-stimulated cell morphogenesis in a β-catenin-dependent manner. Mol. Cell. Biol. 26, 9232–9243 (2006).
O'Brien, L. E. et al. ERK and MMPs sequentially regulate distinct stages of epithelial tubule development. Develop. Cell 7, 21–32 (2004).
Kimura, M. et al. Role of atrophic changes in proximal tubular cells in the peritubular deposition of type IV collagen in a rat renal ablation model. Nephrol. Dial. Transplant. 20, 1559–1565 (2005).
Suzuki, T., Kimura, M., Asano, M., Fujigaki, Y. & Hishida, A. Role of atrophic tubules in development of interstitial fibrosis in microembolism-induced renal failure in rat. Am. J. Pathol. 158, 75–85 (2001).
de Borst, M. H. et al. C-Jun NH2-terminal kinase is crucially involved in renal tubulo-interstitial inflammation. J. Pharmacol. Exp. Ther. 331, 896–905 (2009).
Venkatachalam, M. A. et al. Acute kidney injury: a springboard for progression in chronic kidney disease. Am. J. Physiol. Renal Physiol. 298, F1078–F1094 (2010).
Bomsztyk, K. & Denisenko, O. Epigenetic alterations in acute kidney injury. Semin. Nephrol. 33, 327–340 (2013).
Wing, M. R., Ramezani, A., Gill, H. S., Devaney, J. M. & Raj, D. S. Epigenetics of progression of chronic kidney disease: fact or fantasy? Semin. Nephrol. 33, 363–374 (2013).
Naito, M., Zager, R. A. & Bomsztyk, K. BRG1 increases transcription of proinflammatory genes in renal ischemia. J. Am. Soc. Nephrol. 20, 1787–1796 (2009).
Zager, R. A. & Johnson, A. C. Renal ischemia-reperfusion injury upregulates histone-modifying enzyme systems and alters histone expression at proinflammatory/profibrotic genes. Am. J. Physiol. Renal Physiol. 296, F1032–F1041 (2009).
Yu, J., Feng, Q., Ruan, Y., Komers, R., Kiviat, N. & Bomsztyk, K. Microplate-based platform for combined chromatin and DNA methylation immunoprecipitation assays. BMC Mol. Biol. 12, 49 (2011).
Bonventre, J. V. Kidney injury molecule-1 (Kim-1): a urinary biomarker and much more. Nephrol. Dial. Transplant. 24, 3265–3268 (2009).
Ichimura, T. et al. Kidney injury molecule-1 (Kim-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 273, 4135–4142 (1998).
Ichimura, T. et al. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Invest. 118, 1657–1668 (2008).
Humphreys, B. D. et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J. Clin. Invest. 123, 4023–4035 (2013).
Yang, L. et al. KIM-1/TIM-1 mediated-phagocytosis reduces acute injury to the kidney. J. Clin. Invest. (in press).
Bonventre, J. V. & Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Invest. 121, 4210–4221 (2011).
Mederacke, I. et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nature Commun. 4, 2823 (2013).
Williams, S. M. et al. Class I HDACs regulate angiotensin ii-dependent cardiac fibrosis via fibroblasts and circulating fibrocytes. J. Mol. Cell. Cardiol. 67, 112–125 (2014).
Sahebally, S. M. et al. Circulating fibrocytes and Crohn's disease. Br. J. Surg. 100, 1549–1556 (2013).
Wada, T. et al. Involvement of bone-marrow-derived cells in kidney fibrosis. Clin. Exp. Nephrology. 15, 8–13 (2011).
Ross, R., Everett, N. B. & Tyler, R. Wound healing and collagen formation. VI. The origin of the wound fibroblast studied in parabiosis. J. Cell Biol. 44, 645–654 (1970).
Dulauroy, S., Di Carlo, S. E., Langa, F., Eberl, G. & Peduto, L. Lineage tracing and genetic ablation of adam12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat. Med. 18, 1262–1270 (2012).
Lin, S. L., Kisseleva, T., Brenner, D. A. & Duffield, J. S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 173, 1617–1627 (2008).
Zeisberg, M. & Duffield, J. S. Resolved: EMT produces fibroblasts in the kidney. J. Am. Soc. Nephrol. 21, 1247–1253 (2010).
Iwano, M. et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Invest. 110, 341–350 (2002).
Lin, S. L. et al. Targeting endothelium–pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am. J. Pathol. 178, 911–923 (2011).
Smith, S. W., Chand, S. & Savage, C. O. Biology of the renal pericyte. Nephrol. Dial. Transplant. 27, 2149–2155 (2012).
Betsholtz, C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 15, 215–228 (2004).
Sundberg, C., Kowanetz, M., Brown, L. F., Detmar, M. & Dvorak, H. F. Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab. Invest. 82, 387–401 (2002).
Carvalho, R. L. et al. Defective paracrine signalling by TGFβ in yolk sac vasculature of endoglin mutant mice: A paradigm for hereditary haemorrhagic telangiectasia. Development 131, 6237–6247 (2004).
Benjamin, L. E., Hemo, I. & Keshet, E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-b and VEGF. Development 125, 1591–1598 (1998).
Chae, S. S., Paik, J. H., Allende, M. L., Proia, R. L. & Hla, T. Regulation of limb development by the sphingosine 1-phosphate receptor S1p1/EDG-1 occurs via the hypoxia/VEGF axis. Dev. Biol. 268, 441–447 (2004).
Chen, Y. T. et al. Platelet-derived growth factor receptor signaling activates pericyte–myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 80, 1170–1181 (2011).
Kramann, R. et al. Perivascular gli1(+) progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16, 51–66 (2015).
Leung, K. C., Tonelli, M. & James, M. T. Chronic kidney disease following acute kidney injury-risk and outcomes. Nat. Rev. Nephrol. 9, 77–85 (2013).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Clements, M. E., Chaber, C. J., Ledbetter, S. R. & Zuk, A. Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS ONE 8, e70464 (2013).
Tabibian, J. H., O'Hara, S. P., Splinter, P. L., Trussoni, C. E. & Larusso, N. F. Cholangiocyte senescence via N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology 59, 2263–2275 (2014).
Marongiu, F., Serra, M. P., Sini, M., Angius, F. & Laconi, E. Clearance of senescent hepatocytes in a neoplastic-prone microenvironment delays the emergence of hepatocellular carcinoma. Aging 6, 26–34 (2014).
Westhoff, J. H. et al. Hypertension induces somatic cellular senescence in rats and humans by induction of cell cycle inhibitor p16ink4a. Hypertension 52, 123–129 (2008).
Liu, J. et al. Accelerated senescence of renal tubular epithelial cells is associated with disease progression of patients with immunoglobulin A (IgA) nephropathy. Transl. Res. 159, 454–463 (2012).
Verzola, D. et al. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am. J. Physiol. Renal Physiol. 295, F1563–F1573 (2008).
Franceschi, C. et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. NY Acad. Sci. 908, 244–254 (2000).
Oien, C. M. et al. Living donor kidney transplantation: the effects of donor age and gender on short- and long-term outcomes. Transplantation 83, 600–606 (2007).
Reutzel-Selke, A. et al. Donor age intensifies the early immune response after transplantation. Kidney Int. 71, 629–636 (2007).
Kelly, J., Ali Khan, A., Yin, J., Ferguson, T. A. & Apte, R. S. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J. Clin. Invest. 117, 3421–3426 (2007).
Schmitt, R., Marlier, A. & Cantley, L. G. Zag expression during aging suppresses proliferation after kidney injury. J. Am. Soc. Nephrol. 19, 2375–2383 (2008).
Kwekel, J. C., Desai, V. G., Moland, C. L., Vijay, V. & Fuscoe, J. C. Life cycle analysis of kidney gene expression in male f344 rats. PLoS ONE 8, e75305 (2013).
Kuilman, T., Michaloglou, C., Mooi, W. J. & Peeper, D. S. The essence of senescence. Genes Dev. 24, 2463–2479 (2010).
Itahana, K., Campisi, J. & Dimri, G. P. Methods to detect biomarkers of cellular senescence: the senescence-associated β-galactosidase assay. Methods Mol. Biol. 371, 21–31 (2007).
Yang, H. & Fogo, A. B. Cell senescence in the aging kidney. J. Am. Soc. Nephrol. 21, 1436–1439 (2010).
Megyesi, J., Andrade, L., Vieira, J. M. Jr, Safirstein, R. L. & Price, P. M. Positive effect of the induction of p21WAF1/CIP1 on the course of ischemic acute renal failure. Kidney Int. 60, 2164–2172 (2001).
Dirocco, D. et al. CDK4/6 inhibition induces epithelial cell cycle arrest and ameliorates acute kidney injury. Am. J. Physiol. Renal Physiol. 306, F379–F388 (2013).
Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005).
Loffredo, F. S. et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153, 828–839 (2013).
Villeda, S. A. et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94 (2011).
Baker, D. J. et al. Clearance of p16(Ink4a)-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Yang, H. C. et al. Cells derived from young bone marrow alleviate renal aging. J. Am. Soc. Nephrol. 22, 2028–2036 (2011).
Devarajan, P. Update on mechanisms of ischemic acute kidney injury. J. Am. Soc. Nephrol. 17, 1503–1520 (2006).
Shurin, G. V. et al. Dynamic alteration of soluble serum biomarkers in healthy aging. Cytokine 39, 123–129 (2007).
Kang, D. H. et al. Impaired angiogenesis in the aging kidney: vascular endothelial growth factor and thrombospondin-1 in renal disease. Am. J. Kidney Dis. 37, 601–611 (2001).
Ruiz-Torres, M. P. et al. Age-related increase in expression of TGF-β1 in the rat kidney: relationship to morphologic changes. J. Am. Soc. Nephrol. 9, 782–791 (1998).
Karam, Z. & Tuazon, J. Anatomic and physiologic changes of the aging kidney. Clin. Geriatr. Med. 29, 555–564 (2013).
Poulsom, R. et al. Bone marrow contributes to renal parenchymal turnover and regeneration. J. Pathol. 195, 229–235 (2001).
Duffield, J. S. et al. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J. Clin. Invest. 115, 1743–1755 (2005).
Morigi, M. et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J. Am. Soc. Nephrol. 15, 1794–1804 (2004).
Semedo, P. et al. Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int. Immunopharmacol. 9, 677–682 (2009).
Hagiwara, M., Shen, B., Chao, L. & Chao, J. Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation. Hum. Gene Ther. 19, 807–819 (2008).
Humphreys, B. D. & Bonventre, J. V. Mesenchymal stem cells in acute kidney injury. Annu. Rev. Med. 59, 311–325 (2008).
Thum, T. et al. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ. Res. 100, 434–443 (2007).
Behrens, A., van Deursen, J. M., Rudolph, K. L. & Schumacher, B. Impact of genomic damage and ageing on stem cell function. Nat. Cell Biol. 16, 201–207 (2014).
Stolzing, A. & Scutt, A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell 5, 213–224 (2006).
Schatteman, G. C. & Ma, N. Old bone marrow cells inhibit skin wound vascularization. Stem Cells 24, 717–721 (2006).
Gewin, L. & Zent, R. How does TGF-β mediate tubulointerstitial fibrosis? Semin. Nephrol. 32, 228–235 (2012).
Qi, W., Chen, X., Poronnik, P. & Pollock, C. A. Transforming growth factor-beta/connective tissue growth factor axis in the kidney. Int. J. Biochem. Cell Biol. 40, 9–13 (2008).
Acosta, J. C. et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133, 1006–1018 (2008).
Wu, C. F. et al. Transforming growth factor β-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am. J. Pathol. 182, 118–131 (2013).
Tang, J. et al. Sustained activation of EGFR triggers renal fibrogenesis after acute kidney injury. Am. J. Pathol. 183, 160–172 (2013).
Cianciolo Cosentino, C. et al. Histone deacetylase inhibitor enhances recovery after AKI. J. Am. Soc. Nephrol. 24, 943–953 (2013).
Zhou, W. et al. FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair. Nat. Genet. 44, 910–915 (2012).
Yang, H. C. et al. Cells derived from young bone marrow alleviate renal aging. J. Am. Soc. Nephrol. 22, 2028–2036 (2011).
Kramann, R. & Humphreys, B. D. Kidney pericytes: roles in regeneration and repair. Semin. Nephrol. 34, 374–383 (2014).
Duffield, J. S. & Lupher, M. L. Jr. PRM-151 (recombinant human serum amyloid p/pentraxin 2) for the treatment of fibrosis. Drug News Perspect. 23, 305–315 (2010).
Zhou, L. et al. Activation of p53 promotes renal injury in acute aristolochic acid nephropathy. J. Am. Soc. Nephrol. 21, 31–41 (2010).
Crose, L. E. et al. Alveolar rhabdomyosarcoma-associated PAX3–FOXO1 promotes tumorigenesis via hippo pathway suppression. J. Clin. Invest. 124, 285–296 (2014).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to researching data for the article, discussion of its content, writing and reviewing and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.V.B is co-inventor on KIM-1 patents that are assigned to Partners HealthCare and licensed to Johnson & Johnson, Sekisui, Novartis, Biogen Idec., R & D, and Astute. He is a consultant for Astellas, Novartis, Roche and Sekisui regarding the safety and efficacy of therapeutics or diagnostics for acute kidney injury. He holds equity in MediBeacon, Sentien and Thrasos, and has grant support from Novo Nordisk and Roche. D.A.F declares no competing interests.
Rights and permissions
About this article
Cite this article
Ferenbach, D., Bonventre, J. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 11, 264–276 (2015). https://doi.org/10.1038/nrneph.2015.3
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2015.3
This article is cited by
-
Critical roles of tubular mitochondrial ATP synthase dysfunction in maleic acid-induced acute kidney injury
Apoptosis (2024)
-
Pre-clinical evaluation of biomarkers for the early detection of nephrotoxicity following alpha-particle radioligand therapy
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
Epidemiology of acute kidney injury in children: a report from the 26th Acute Disease Quality Initiative (ADQI) consensus conference
Pediatric Nephrology (2024)
-
Hexarelin alleviates apoptosis on ischemic acute kidney injury via MDM2/p53 pathway
European Journal of Medical Research (2023)
-
The gut microbiome tango in the progression of chronic kidney disease and potential therapeutic strategies
Journal of Translational Medicine (2023)