Key Points
-
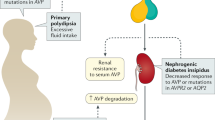
Nephrogenic diabetes insipidus (NDI) is caused by inability of the kidneys to concentrate urine by reabsorbing water in the collecting duct
-
NDI can be inherited (X-linked or autosomal) or acquired, most commonly as a result of lithium treatment
-
Management of primary forms of NDI focuses on dietary modification to reduce osmotic load and pharmacological treatment with inhibitors of prostaglandin synthesis and thiazide diuretics
-
With appropriate treatment, complications of NDI—such as failure to thrive and mental retardation resulting from repeated hypernatraemic dehydration—can be avoided
-
New treatment approaches for congenital NDI have been tested in animal models, but efficacy in patients has not yet been confirmed
Abstract
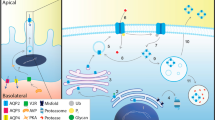
Healthy kidneys maintain fluid and electrolyte homoeostasis by adjusting urine volume and composition according to physiological needs. The final urine composition is determined in the last tubular segment: the collecting duct. Water permeability in the collecting duct is regulated by arginine vasopressin (AVP). Secretion of AVP from the neurohypophysis is regulated by a complex signalling network that involves osmosensors, barosensors and volume sensors. AVP facilitates aquaporin (AQP)-mediated water reabsorption via activation of the vasopressin V2 receptor (AVPR2) in the collecting duct, thus enabling concentration of urine. In nephrogenic diabetes insipidus (NDI), inability of the kidneys to respond to AVP results in functional AQP deficiency. Consequently, affected patients have constant diuresis, resulting in large volumes of dilute urine. Primary forms of NDI result from mutations in the genes that encode the key proteins AVPR2 and AQP2, whereas secondary forms are associated with biochemical abnormalities, obstructive uropathy or the use of certain medications, particularly lithium. Treatment of the disease is informed by identification of the underlying cause. Here we review the clinical aspects and diagnosis of NDI, the various aetiologies, current treatment options and potential future developments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Stevens, L. A., Coresh, J., Greene, T. & Levey, A. S. Assessing kidney function—measured and estimated glomerular filtration rate. N. Engl. J. Med. 354, 2473–2483 (2006).
Nielsen, S., Marples, D., Frokiaer, J., Knepper, M. & Agre, P. The aquaporin family of water channels in kidney: an update on physiology and pathophysiology of aquaporin-2. Kidney Int. 49, 1718–1723 (1996).
Nielsen, S. et al. Aquaporin-1 water channels in short and long loop descending thin limbs and in descending vasa recta in rat kidney. Am. J. Physiol. 268, F1023–F1037 (1995).
Dantzler, W. H., Layton, A. T., Layton, H. E. & Pannabecker, T. L. Urine-concentrating mechanism in the inner medulla: function of the thin limbs of the loops of Henle. Clin. J. Am. Soc. Nephrol. 9, 1781–1789 (2014).
Halperin, M. L., Kamel, K. S. & Oh, M. S. Mechanisms to concentrate the urine: an opinion. Curr. Opin. Nephrol. Hypertens. 17, 416–422 (2008).
Pannabecker, T. L. Structure and function of the thin limbs of the loop of Henle. Compr. Physiol. 2, 2063–2086 (2012).
Obermuller, N., Kunchaparty, S., Ellison, D. H. & Bachmann, S. Expression of the Na-K-2Cl cotransporter by macula densa and thick ascending limb cells of rat and rabbit nephron. J. Clin. Invest. 98, 635–640 (1996).
Bichet, D. G., Oksche, A. & Rosenthal, W. Congenital nephrogenic diabetes insipidus. J. Am. Soc. Nephrol. 8, 1951–1958 (1997).
Nielsen, S. et al. Aquaporins in the kidney: from molecules to medicine. Physiol. Rev. 82, 205–244 (2002).
Kortenoeven, M. L. & Fenton, R. A. Renal aquaporins and water balance disorders. Biochim. Biophys. Acta 1840, 1533–1549 (2014).
Fushimi, K., Sasaki, S. & Marumo, F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J. Biol. Chem. 272, 14800–14804 (1997).
Kamsteeg, E. J., Heijnen, I., van Os, C. H. & Deen, P. M. The subcellular localization of an aquaporin-2 tetramer depends on the stoichiometry of phosphorylated and nonphosphorylated monomers. J. Cell Biol. 151, 919–930 (2000).
Mulders, S. M. et al. An aquaporin-2 water channel mutant which causes autosomal dominant nephrogenic diabetes insipidus is retained in the Golgi complex. J. Clin. Invest. 102, 57–66 (1998).
Savelkoul, P. J. et al. p.R254Q mutation in the aquaporin-2 water channel causing dominant nephrogenic diabetes insipidus is due to a lack of arginine vasopressin-induced phosphorylation. Hum. Mutat. 30, E891–E903 (2009).
Hoffert, J. D. et al. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J. Biol. Chem. 283, 24617–24627 (2008).
Moeller, H. B., Praetorius, J., Rutzler, M. R. & Fenton, R. A. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc. Natl Acad. Sci USA 107, 424–429 (2010).
Tamma, G., Robben, J. H., Trimpert, C., Boone, M. & Deen, P. M. Regulation of AQP2 localization by S256 and S261 phosphorylation and ubiquitination. Am. J. Physiol. Cell Physiol. 300, C636–C646 (2011).
Kamsteeg, E. J. et al. Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc. Natl Acad. Sci. USA 103, 18344–18349 (2006).
Rehmann, H., Wittinghofer, A. & Bos, J. L. Capturing cyclic nucleotides in action: snapshots from crystallographic studies. Nat. Rev. Mol. Cell Biol. 8, 63–73 (2007).
Holz, G. G., Kang, G., Harbeck, M., Roe, M. W. & Chepurny, O. G. Cell physiology of cAMP sensor Epac. J. Physiol. 577, 5–15 (2006).
Kortenoeven, M. L. et al. In mpkCCD cells, long-term regulation of aquaporin-2 by vasopressin occurs independent of protein kinase A and CREB but may involve Epac. Am. J. Physiol. Renal Physiol. 302, F1395–F1401 (2012).
Hozawa, S., Holtzman, E. J. & Ausiello, D. A. cAMP motifs regulating transcription in the aquaporin 2 gene. Am. J. Physiol. 270, C1695–C1702 (1996).
Matsumura, Y., Uchida, S., Rai, T., Sasaki, S. & Marumo, F. Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J. Am. Soc. Nephrol. 8, 861–867 (1997).
Yasui, M., Zelenin, S. M., Celsi, G. & Aperia, A. Adenylate cyclase-coupled vasopressin receptor activates AQP2 promoter via a dual effect on CRE and AP1 elements. Am. J. Physiol. 272, F443–F450 (1997).
Hasler, U. et al. Long term regulation of aquaporin-2 expression in vasopressin-responsive renal collecting duct principal cells. J. Biol. Chem. 277, 10379–10386 (2002).
Terris, J., Ecelbarger, C. A., Nielsen, S. & Knepper, M. A. Long-term regulation of four renal aquaporins in rats. Am. J. Physiol. 271, F414–F422 (1996).
Lolait, S. J. et al. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature 357, 336–339 (1992).
Rosenthal, W. et al. Molecular identification of the gene responsible for congenital nephrogenic diabetes insipidus. Nature 359, 233–235 (1992).
van den Ouweland, A. M. et al. Mutations in the vasopressin type 2 receptor gene (AVPR2) associated with nephrogenic diabetes insipidus. Nat. Genet. 2, 99–102 (1992).
Pan, Y., Metzenberg, A., Das, S., Jing, B. & Gitschier, J. Mutations in the V2 vasopressin receptor gene are associated with X-linked nephrogenic diabetes insipidus. Nat. Genet. 2, 103–106 (1992).
Fushimi, K. et al. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361, 549–552 (1993).
Sasaki, S. et al. Cloning, characterization, and chromosomal mapping of human aquaporin of collecting duct. J. Clin. Invest. 93, 1250–1256 (1994).
Deen, P. M. et al. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science 264, 92–95 (1994).
Sasaki, S., Chiga, M., Kikuchi, E., Rai, T. & Uchida, S. Hereditary nephrogenic diabetes insipidus in Japanese patients: analysis of 78 families and report of 22 new mutations in AVPR2 and AQP2. Clin. Exp. Nephrol. 17, 338–344 (2013).
Arthus, M. F. et al. Report of 33 novel AVPR2 mutations and analysis of 117 families with X-linked nephrogenic diabetes insipidus. J. Am. Soc. Nephrol. 11, 1044–1054 (2000).
Sands, J. M. & Bichet, D. G. Nephrogenic diabetes insipidus. Ann. Intern. Med. 144, 186–194 (2006).
Wesche, D., Deen, P. M. & Knoers, N. V. Congenital nephrogenic diabetes insipidus: the current state of affairs. Pediatr. Nephrol. 27, 2183–2204 (2012).
Bichet, D. G. Nephrogenic diabetes insipidus. Adv. Chronic Kidney Dis. 13, 96–104 (2006).
van Lieburg, A. F., Knoers, N. V. & Monnens, L. A. Clinical presentation and follow-up of 30 patients with congenital nephrogenic diabetes insipidus. J. Am. Soc. Nephrol. 10, 1958–1964 (1999).
Sato, K. et al. A novel mutation in the vasopressin V2 receptor gene in a woman with congenital nephrogenic diabetes insipidus. Intern. Med. 38, 808–812 (1999).
Kinoshita, K. et al. A novel deletion mutation in the arginine vasopressin receptor 2 gene and skewed X chromosome inactivation in a female patient with congenital nephrogenic diabetes insipidus. J. Endocrinol. Invest. 27, 167–170 (2004).
Satoh, M., Ogikubo, S. & Yoshizawa-Ogasawara, A. Correlation between clinical phenotypes and X-inactivation patterns in six female carriers with heterozygote vasopressin type 2 receptor gene mutations. Endocr. J. 55, 277–284 (2008).
Bockenhauer, D. et al. Vasopressin type 2 receptor V88M mutation: molecular basis of partial and complete nephrogenic diabetes insipidus. Nephron Physiol. 114, 1–10 (2010).
Bichet, D. G. et al. X-linked nephrogenic diabetes insipidus mutations in North America and the Hopewell hypothesis. J. Clin. Invest. 92, 1262–1268 (1993).
Fujiwara, T. M. & Bichet, D. G. Molecular biology of hereditary diabetes insipidus. J. Am. Soc. Nephrol. 16, 2836–2846 (2005).
Spanakis, E., Milord, E. & Gragnoli, C. AVPR2 variants and mutations in nephrogenic diabetes insipidus: review and missense mutation significance. J. Cell Physiol. 217, 605–617 (2008).
Morello, J. P. et al. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J. Clin. Invest. 105, 887–895 (2000).
Duzenli, D. et al. Mutations in the AVPR2, AVP-NPII, and AQP2 genes in Turkish patients with diabetes insipidus. Endocrine 42, 664–669 (2012).
Marr, N. et al. Heteroligomerization of an aquaporin-2 mutant with wild-type aquaporin-2 and their misrouting to late endosomes/lysosomes explains dominant nephrogenic diabetes insipidus. Hum. Mol. Genet. 11, 779–789 (2002).
Kuwahara, M. et al. Three families with autosomal dominant nephrogenic diabetes insipidus caused by aquaporin-2 mutations in the C-terminus. Am. J. Hum. Genet. 69, 738–748 (2001).
Kamsteeg, E. J. et al. Reversed polarized delivery of an aquaporin-2 mutant causes dominant nephrogenic diabetes insipidus. J. Cell Biol. 163, 1099–1109 (2003).
Frick, A. et al. X-ray structure of human aquaporin 2 and its implications for nephrogenic diabetes insipidus and trafficking. Proc. Natl Acad. Sci USA 111, 6305–6310 (2014).
Bichet, D. G. et al. Aquaporin-2: new mutations responsible for autosomal-recessive nephrogenic diabetes insipidus—update and epidemiology. Clin. Kidney J. 5, 195–202 (2012).
Bockenhauer, D. et al. Secondary nephrogenic diabetes insipidus as a complication of inherited renal diseases. Nephron Physiol. 116, 23–29 (2010).
Bockenhauer, D. & Bichet, D. G. Inherited secondary nephrogenic diabetes insipidus: concentrating on humans. Am. J. Physiol. Renal Physiol. 304, F1037–F1042 (2013).
Bettinelli, A. et al. Phenotypic variability in Bartter syndrome type I. Pediatr. Nephrol. 14, 940–945 (2000).
Hebert, S. C., Brown, E. M. & Harris, H. W. Role of the Ca2+-sensing receptor in divalent mineral ion homeostasis. J. Exp. Biol. 200, 295–302 (1997).
Marples, D., Frokiaer, J., Dorup, J., Knepper, M. A. & Nielsen, S. Hypokalemia-induced downregulation of aquaporin-2 water channel expression in rat kidney medulla and cortex. J. Clin. Invest. 97, 1960–1968 (1996).
Sands, J. M. et al. Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct. J. Clin. Invest. 99, 1399–1405 (1997).
Earm, J. H. et al. Decreased aquaporin-2 expression and apical plasma membrane delivery in kidney collecting ducts of polyuric hypercalcemic rats. J. Am. Soc. Nephrol. 9, 2181–2193 (1998).
Trepiccione, F. & Christensen, B. M. Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J. Nephrol. 23 (Suppl. 16), S43–S48 (2010).
Rej, S., Herrmann, N. & Shulman, K. The effects of lithium on renal function in older adults—a systematic review. J. Geriatr. Psychiatry Neurol. 25, 51–61 (2012).
Timmer, R. T. & Sands, J. M. Lithium intoxication. J. Am. Soc. Nephrol. 10, 666–674 (1999).
Hetmar, O. et al. Lithium: long-term effects on the kidney. I. Renal function in retrospect. Acta Psychiatr. Scand. 73, 574–581 (1986).
Juurlink, D. N. et al. Drug-induced lithium toxicity in the elderly: a population-based study. J. Am. Geriatr. Soc. 52, 794–798 (2004).
Head, L. & Dening, T. Lithium in the over-65s: who is taking it and who is monitoring it? A survey of older adults on lithium in the Cambridge Mental Health Services catchment area. Int. J. Geriatr. Psychiatry 13, 164–171 (1998).
Christensen, B. M. et al. Changes in cellular composition of kidney collecting duct cells in rats with lithium-induced NDI. Am. J. Physiol. Cell Physiol. 286, C952–C964 (2004).
Li, Y., Shaw, S., Kamsteeg, E. J., Vandewalle, A. & Deen, P. M. Development of lithium-induced nephrogenic diabetes insipidus is dissociated from adenylyl cyclase activity. J. Am. Soc. Nephrol. 17, 1063–1072 (2006).
Walker, R. J. et al. Lithium-induced reduction in urinary concentrating ability and urinary aquaporin 2 (AQP2) excretion in healthy volunteers. Kidney Int. 67, 291–294 (2005).
Kortenoeven, M. L. et al. Amiloride blocks lithium entry through the sodium channel thereby attenuating the resultant nephrogenic diabetes insipidus. Kidney Int. 76, 44–53 (2009).
Bedford, J. J. et al. Lithium-induced nephrogenic diabetes insipidus: renal effects of amiloride. Clin. J. Am. Soc. Nephrol. 3, 1324–1331 (2008).
Batlle, D. C., von Riotte, A. B., Gaviria, M. & Grupp, M. Amelioration of polyuria by amiloride in patients receiving long-term lithium therapy. N. Engl. J. Med. 312, 408–414 (1985).
Frokiaer, J., Marples, D., Knepper, M. A. & Nielsen, S. Bilateral ureteral obstruction downregulates expression of vasopressin-sensitive AQP-2 water channel in rat kidney. Am. J. Physiol. 270, F657–F668 (1996).
Frokiaer, J. et al. Downregulation of aquaporin-2 parallels changes in renal water excretion in unilateral ureteral obstruction. Am. J. Physiol. 273, F213–F223 (1997).
Winberg, J. Determination of renal concentration capacity in infants and children without renal disease. Acta Paediatrica 48, 318–328 (1958).
Bichet, D. G. et al. Hemodynamic and coagulation responses to 1-desamino[8-D-arginine] vasopressin in patients with congenital nephrogenic diabetes insipidus. N. Engl. J. Med. 318, 881–887 (1988).
Mannucci, P. M. Treatment of von Willebrand's Disease. N. Engl. J. Med. 351, 683–694 (2004).
Kaufmann, J. E. & Vischer, U. M. Cellular mechanisms of the hemostatic effects of desmopressin (DDAVP). J. Thromb. Haemost. 1, 682–689 (2003).
Hillman, D. A., Neyzi, O., Porter, P., Cushman, A. & Talbot, N. B. Renal (vasopressin-resistant) diabetes insipidus; definition of the effects of a homeostatic limitation in capacity to conserve water on the physical, intellectual and emotional development of a child. Pediatrics 21, 430–435 (1958).
Vest, M., Talbot. N. B. & Crawford, J. D. Hypocaloric dwarfism and hydronephrosis in diabetes insipidus. Am. J. Dis. Child 105, 175–181 (1963).
Schofer, O. et al. Nephrogenic diabetes insipidus and intracerebral calcification. Arch. Dis. Child 65, 885–887 (1990).
Hoekstra, J. A., van Lieburg, A. F., Monnens, L. A., Hulstijn-Dirkmaat, G. M. & Knoers, V. V. Cognitive and psychosocial functioning of patients with congenital nephrogenic diabetes insipidus. Am. J. Med. Genet. 61, 81–88 (1996).
Huber, D., Veinante, P. & Stoop, R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 308, 245–248 (2005).
Griebel, G. et al. Anxiolytic and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc. Natl Acad. Sci USA 99, 6370–6375 (2002).
Yoo, T. H. et al. Congenital nephrogenic diabetes insipidus presented with bilateral hydronephrosis: genetic analysis of V2R gene mutations. Yonsei Med. J. 47, 126–130 (2006).
Stevens, S., Brown, B. D. & McGahan, J. P. Nephrogenic diabetes insipidus: a cause of severe nonobstructive urinary tract dilatation. J. Ultrasound Med. 14, 543–545 (1995).
Jaureguiberry, G. et al. A patient with polyuria and hydronephrosis: question. Pediatr. Nephrol. 26, 1977–1978 (2011).
Sadeghi, H., Robertson, G. L., Bichet, D. G., Innamorati, G. & Birnbaumer, M. Biochemical basis of partial nephrogenic diabetes insipidus phenotypes. Mol. Endocrinol. 11, 1806–1813 (1997).
Canfield, M. C., Tamarappoo, B. K., Moses, A. M., Verkman, A. S. & Holtzman, E. J. Identification and characterization of aquaporin-2 water channel mutations causing nephrogenic diabetes insipidus with partial vasopressin response. Hum. Mol. Genet. 6, 1865–1871 (1997).
Guyon, C. et al. Characterization of D150E and G196D aquaporin-2 mutations responsible for nephrogenic diabetes insipidus: importance of a mild phenotype. Am. J. Physiol. Renal Physiol. 297, F489–F498 (2009).
Bichet, D. G. et al. Nature and recurrence of AVPR2 mutations in X-linked nephrogenic diabetes insipidus. Am. J. Hum. Genet. 55, 278–286 (1994).
Ala, Y. et al. Functional studies of twelve mutant V2 vasopressin receptors related to nephrogenic diabetes insipidus: molecular basis of a mild clinical phenotype. J. Am. Soc. Nephrol. 9, 1861–1872 (1998).
Kahn, A., Brachet, E. & Blum, D. Controlled fall in natremia and risk of seizures in hypertonic dehydration. Intensive Care Med. 5, 27–31 (1979).
Cansick, J., Rees, L., Koffman, G., Van' t Hoff, W. & Bockenhauer, D. A fatal case of cerebral oedema with hyponatraemia and massive polyuria after renal transplantation. Pediatr. Nephrol. 24, 1231–1234 (2009).
Sterns, R. H. Disorders of plasma sodium—causes, consequences, and correction. N. Engl. J. Med. 372, 55–65 (2015).
Fang, C. et al. Fluid management of hypernatraemic dehydration to prevent cerebral oedema: a retrospective case control study of 97 children in China. J. Paediatr. Child Health 46, 301–303 (2010).
Meyer, E. Über diabetes insipidus und andere polyurien [German]. Arch. Klin. Med. 83, 1 (1905).
Crawford, J. D. & Kennedy, G. C. Chlorothiazid in diabetes insipidus. Nature 183, 891–892 (1959).
Havard, C. W. Thiazide-induced antidiuresis in diabetes insipidus. Proc. R. Soc. Med. 58, 1005–1007 (1965).
Sinke, A. P. et al. Hydrochlorothiazide attenuates lithium-induced nephrogenic diabetes insipidus independently of the sodium-chloride cotransporter. Am. J. Physiol. Renal Physiol. 306, F525–F533 (2014).
Orloff, J., Handler, J. S. & Bergstrom, S. Effect of [prostaglandin (Pge-1) on the permeability response of toad bladder to vasopressin, theophylline and adenosine 3′,5′-monophosphate. Nature 205, 397–398 (1965).
Anderson, R. J., Berl, T., McDonald, K. D. & Schrier, R. W. Evidence for an in vivo antagonism between vasopressin and prostaglandin in the mammalian kidney. J. Clin. Invest. 56, 420–426 (1975).
Stoff, J. S., Rosa, R. M., Silva, P. & Epstein, F. H. Indomethacin impairs water diuresis in the DI rat: role of prostaglandins independent of ADH. Am. J. Physiol. 241, F231–F237 (1981).
Libber, S., Harrison, H. & Spector, D. Treatment of nephrogenic diabetes insipidus with prostaglandin synthesis inhibitors. J. Pediatr. 108, 305–311 (1986).
Monnens, L., Jonkman, A. & Thomas, C. Response to indomethacin and hydrochlorothiazide in nephrogenic diabetes insipidus. Clin. Sci. (Lond.) 66, 709–715 (1984).
Monn, E. Prostaglandin synthetase inhibitors in the treatment of nephrogenic diabetes insipidus. Acta Paediatr. Scand. 70, 39–42 (1981).
Usberti, M. et al. Renal prostaglandin E2 in nephrogenic diabetes insipidus: effects of inhibition of prostaglandin synthesis by indomethacin. J. Pediatr. 97, 476–478 (1980).
Boussemart, T., Nsota, J., Martin-Coignard, D. & Champion, G. Nephrogenic diabetes insipidus: treat with caution. Pediatr. Nephrol. 24, 1761–1763 (2009).
Bockenhauer, D. et al. Antenatal Bartter's syndrome: why is this not a lethal condition? QJM 101, 927–942 (2008).
Bockenhauer, D., Medlar, A. J., Ashton, E., Kleta, R. & Lench, N. Genetic testing in renal disease. Pediatr. Nephrol. 27, 873–883 (2012).
Bernier, V. et al. Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. J. Am. Soc. Nephrol. 17, 232–243 (2006).
Erdelyi, L. S. et al. Altered agonist sensitivity of a mutant v2 receptor suggests a novel therapeutic strategy for nephrogenic diabetes insipidus. Mol. Endocrinol. 28, 634–643 (2014).
Li, J. H. et al. A selective EP4 PGE2 receptor agonist alleviates disease in a new mouse model of X-linked nephrogenic diabetes insipidus. J. Clin. Invest. 119, 3115–3126 (2009).
Olesen, E. T., Rutzler, M. R., Moeller, H. B., Praetorius, H. A. & Fenton, R. A. Vasopressin-independent targeting of aquaporin-2 by selective E-prostanoid receptor agonists alleviates nephrogenic diabetes insipidus. Proc. Natl Acad. Sci. USA 108, 12949–12954 (2011).
Bockenhauer, D. & Bichet, D. G. Urinary concentration: different ways to open and close the tap. Pediatr. Nephrol. 29, 1297–1303 (2014).
Chu, J. Y. et al. Phenotypes developed in secretin receptor-null mice indicated a role for secretin in regulating renal water reabsorption. Mol. Cell Biol. 27, 2499–2511 (2007).
Procino, G. et al. Combination of secretin and fluvastatin ameliorates the polyuria associated with X-linked nephrogenic diabetes insipidus in mice. Kidney Int. 86, 127–138 (2014).
Bouley, R. et al. Stimulation of AQP2 membrane insertion in renal epithelial cells in vitro and in vivo by the cGMP phosphodiesterase inhibitor sildenafil citrate (Viagra). Am. J. Physiol. Renal Physiol. 288, F1103–F1112 (2005).
Ran, F. A. et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015).
Cyranoski, D. Ethics of embryo editing divides scientists. Nature 519, 272 (2015).
Oka, Y., Ye, M. & Zuker, C. S. Thirst driving and suppressing signals encoded by distinct neural populations in the brain. Nature 520, 349–352 (2015).
Cannon, J. F. Diabetes insipidus; clinical and experimental studies with consideration of genetic relationships. AMA Arch. Intern. Med. 96, 215–272 (1955).
Lacombe, L. De la polydipsie [French]. J. Med. Chir. 7, 323–329 (1841).
McIlraith, C. H. Notes on some cases of diabetes insipidus with marked family and hereditary tendencies. Lancet 2, 767 (1892).
Magnus, R. & Schafer, E. A. Effects of post-pituitary extracts. J. Physiol. 12, 32–38 (1901).
Farini, F. Ueber diabetes insipidus and hypophysis therapie. Wien Klin. Wochenschr. 26, 1867 (1913).
Velden, V. D. Die nierenwirkung von hypophysenextracte beim menschen [German]. Berl. Klin. Wochenschr. 50, 2083 (1913).
de Lange, C. Ueber erblichen diabetes insipidus [German]. Jahrbuch fuer Kinderheilkunde 145, 135 (1935).
Forssman, H. H. On hereditary diabetes insipidus. Acta Medica Scandinavica 121, 9 (1945).
Waring, A. J., Kadji, L. & Tappan, V. A congenital defect of water metabolism. Am. J. Dis. Child. 69, 323–324 (1945).
Williams, R. H. & Henry, C. Nephrogenic diabetes insipidus: transmitted by females and appearing during infancy in males. Ann. Intern. Med. 27, 84–95 (1947).
Coleman, J. in Clinical Paediatric Dietetics 2nd edn (eds Shaw, V. & Lawson, M.) 158–182 (Blackwell Science, 2001).
Acknowledgements
D.B.'s work is supported by the Higher Education Funding Council for England and the European Consortium for High-Throughput Research in Rare Kidney Diseases (grant 2012-305608).
Author information
Authors and Affiliations
Contributions
Both authors researched the data, wrote the article and edited and/or reviewed the manuscript before publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bockenhauer, D., Bichet, D. Pathophysiology, diagnosis and management of nephrogenic diabetes insipidus. Nat Rev Nephrol 11, 576–588 (2015). https://doi.org/10.1038/nrneph.2015.89
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2015.89
This article is cited by
-
HYDROchlorothiazide versus placebo to PROTECT polycystic kidney disease patients and improve their quality of life: study protocol and rationale for the HYDRO-PROTECT randomized controlled trial
Trials (2024)
-
Targeted long-read sequencing identified a causal structural variant in X-linked nephrogenic diabetes insipidus
BMC Medical Genomics (2024)
-
Long-term lithium therapy and risk of chronic kidney disease, hyperparathyroidism and hypercalcemia: a cohort study
International Journal of Bipolar Disorders (2023)
-
Structural and functional analysis of aquaporin-2 mutants involved in nephrogenic diabetes insipidus
Scientific Reports (2023)
-
A case of ifosfamide-induced acute kidney injury, Fanconi syndrome, and nephrogenic diabetes insipidus
CEN Case Reports (2023)