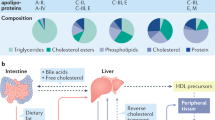
Key Points
-
Prolonged hyperlipidaemia in nephrotic syndrome is a major risk factor for multiple disease complications, including accelerated atherosclerosis, myocardial infarction, stroke, chronic kidney disease and thrombosis
-
Direct lipid-induced cellular injury to podocytes, mesangial cells and, potentially, renal tubular cells as a result of dyslipidaemia increasingly seems to have a role in the pathogenesis of nephrotic syndrome
-
Given the available evidence, we suggest that statins should be the first-line treatment for prolonged hyperlipidaemia in patients with nephrotic syndrome, given their efficacy in the treatment of other diseases and the fact that they are well tolerated
-
Alternative, less supported treatments include LDL apheresis, cholesterol absorption inhibitors, nicotinic acid and bile acid sequestrants; targeting proprotein convertase subtilisin/kexin type 9 is another potential treatment for hyperlipidaemia in patients with nephrotic syndrome
-
Treatment recommendations in children are limited by a lack of data for both the efficacy and the risk of pharmacological interventions
Abstract
Nephrotic syndrome is a highly prevalent disease that is associated with high morbidity despite notable advances in its treatment. Many of the complications of nephrotic syndrome, including the increased risk of atherosclerosis and thromboembolism, can be linked to dysregulated lipid metabolism and dyslipidaemia. These abnormalities include elevated plasma levels of cholesterol, triglycerides and the apolipoprotein B-containing lipoproteins VLDL and IDL; decreased lipoprotein lipase activity in the endothelium, muscle and adipose tissues; decreased hepatic lipase activity; and increased levels of the enzyme PCSK9. In addition, there is an increase in the plasma levels of immature HDL particles and reduced cholesterol efflux. Studies from the past few years have markedly improved our understanding of the molecular pathogenesis of nephrotic syndrome-associated dyslipidaemia, and also heightened our awareness of the associated exacerbated risks of cardiovascular complications, progressive kidney disease and thromboembolism. Despite the absence of clear guidelines regarding treatment, various strategies are being increasingly utilized, including statins, bile acid sequestrants, fibrates, nicotinic acid and ezetimibe, as well as lipid apheresis, which seem to also induce partial or complete clinical remission of nephrotic syndrome in a substantial percentage of patients. Future potential treatments will likely also include inhibition of PCSK9 using recently-developed anti-PCSK9 monoclonal antibodies and small inhibitory RNAs, as well as targeting newly identified molecular regulators of lipid metabolism that are dysregulated in nephrotic syndrome.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
13 December 2017
In the version of this article originally published online, the affiliations of the authors were incorrect. This error has now been corrected in the print and online versions.
References
Greenbaum, L. A., Benndorf, R. & Smoyer, W. E. Childhood nephrotic syndrome — current and future therapies. Nat. Rev. Nephrol. 8, 445–458 (2012).
Hull, R. P. & Goldsmith, D. J. Nephrotic syndrome in adults. BMJ 336, 1185–1189 (2008).
Clark, A. G. & Barratt, T. M. in Pediatric Nephrology (eds Barratt, T. M., Avner, E. D. & Harmon, W. E.) 731–747 (Lippincott Williams & Wilkins, 1998).
McEnery, P. T. & Strife, C. F. Nephrotic syndrome in childhood. Management and treatment in patients with minimal change disease, mesangial proliferation, or focal glomerulosclerosis. Pediatr. Clin. North Am. 89, 875–894 (1982).
Nash, M. A., Edelmann, C. M. J., Bernstein, J. & Barnett, H. L. in Pediatric Kidney Disease (ed. Edelmann, C. M. J.) 1247–1266 (Little, 1992).
Ponticelli, C. et al. Can prolonged treatment improve the prognosis in adults with focal segmental glomerulosclerosis? Am. J. Kidney Dis 34, 618–625 (1999).
MacHardy, N. et al. Management patterns of childhood-onset nephrotic syndrome. Pediatr. Nephrol. 24, 2193–2201 (2009).
Ding, W. Y. & Saleem, M. A. Current concepts of the podocyte in nephrotic syndrome. Kidney Res. Clin. Pract. 31, 87–93 (2012).
Harris, R. C. & Ismail, N. Extrarenal complications of the nephrotic syndrome. Am. J. Kidney Dis 23, 477–497 (1994).
Cameron, J. S. The nephrotic syndrome and its complications. Am. J. Kidney Dis 10, 157–171 (1987).
Llach, F. Hypercoagulability, renal vein thrombosis, and other thrombotic complications of nephrotic syndrome. Kidney Int. 28, 429–439 (1985).
Rheault, M. N. et al. AKI in children hospitalized with nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 10, 2110–2118 (2015).
Al-Azzawi, H. F., Obi, O. C., Safi, J. & Song, M. Nephrotic syndrome-induced thromboembolism in adults. Int. J. Crit. Illn. Inj. Sci. 6, 85–88 (2016).
Kerlin, B. A., Ayoob, R. & Smoyer, W. E. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin. J. Am. Soc. Nephrol. 7, 513–520 (2012).
Kerlin, B. A., Haworth, K. & Smoyer, W. E. Venous thromboembolism in pediatric nephrotic syndrome. Pediatr. Nephrol. 29, 989–997 (2014).
Loscalzo, J. Venous thrombosis in the nephrotic syndrome. N. Engl. J. Med. 368, 956–958 (2013).
Vaziri, N. D. Disorders of lipid metabolism in nephrotic syndrome: mechanisms and consequences. Kidney Int. 90, 41–52 (2016).
Joven, J. et al. Abnormalities of lipoprotein metabolism in patients with the nephrotic syndrome. N. Engl. J. Med. 323, 579–584 (1990).
Zhou, H., Tan, K. C., Shiu, S. W. & Wong, Y. Cellular cholesterol efflux to serum is impaired in diabetic nephropathy. Diabetes Metab. Res. Rev. 24, 617–623 (2008).
de Sain- van der Velden, M. G. et al. Increased VLDL in nephrotic patients results from a decreased catabolism while increased LDL results from increased synthesis. Kidney Int. 53, 994–1001 (1998).
Garber, D. W., Gottlieb, B. A., Marsh, J. B. & Sparks, C. E. Catabolism of very low density lipoproteins in experimental nephrosis. J. Clin. Invest. 74, 1375–1383 (1984).
Davies, R. W., Staprans, I., Hutchison, F. N. & Kaysen, G. A. Proteinuria, not altered albumin metabolism, affects hyperlipidemia in the nephrotic rat. J. Clin. Invest. 86, 600–605 (1990).
Vaziri, N. D. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am. J. Physiol. Renal Physiol. 290, F262–F272 (2006).
Mace, C. & Chugh, S. S. Nephrotic syndrome: components, connections, and angiopoietin-like 4-related therapeutics. J. Am. Soc. Nephrol. 25, 2393–2398 (2014).
Keith, D. S., Nichols, G. A., Gullion, C. M., Brown, J. B. & Smith, D. H. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch. Intern. Med. 164, 659–663 (2004).
Yeang, C., Gordts, P. L. & Tsimikas, S. Novel lipoprotein(a) catabolism pathway via apolipoprotein(a) recycling: Adding the plasminogen receptor PlgRKT to the list. Circ. Res. 120, 1050–1052 (2017).
Sharma, M., Redpath, G. M., Williams, M. J. & McCormick, S. P. Recycling of apolipoprotein(a) after PlgRKT-mediated endocytosis of lipoprotein(a). Circ. Res. 120, 1091–1102 (2017).
Moriarty, P. M., Varvel, S. A., Gordts, P. L., McConnell, J. P. & Tsimikas, S. Lipoprotein(a) mass levels increase significantly according to APOE genotype: an analysis of 431 239 patients. Arterioscler. Thromb. Vasc. Biol. 37, 580–588 (2017).
Merkel, M., Eckel, R. H. & Goldberg, I. J. Lipoprotein lipase: genetics, lipid uptake, and regulation. J. Lipid Res. 43, 1997–2006 (2002).
Allan, C. M. et al. Mobility of “HSPG-bound” LPL explains how LPL is able to reach GPIHBP1 on capillaries. J. Lipid Res. 58, 216–225 (2017).
Davies, B. S. et al. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 12, 42–52 (2010).
Vaziri, N. D., Yuan, J., Ni, Z., Nicholas, S. B. & Norris, K. C. Lipoprotein lipase deficiency in chronic kidney disease is accompanied by down-regulation of endothelial GPIHBP1 expression. Clin. Exp. Nephrol. 16, 238–243 (2012).
Moorhead, J. F., Chan, M. K., El-Nahas, M. & Varghese, Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet 2, 1309–1311 (1982).
Clement, L. C. et al. Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat. Med. 20, 37–46 (2014).
Sukonina, V., Lookene, A., Olivecrona, T. & Olivecrona, G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc. Natl Acad. Sci. USA 103, 17450–17455 (2006).
Lafferty, M. J., Bradford, K. C., Erie, D. A. & Neher, S. B. Angiopoietin-like protein 4 inhibition of lipoprotein lipase: evidence for reversible complex formation. J. Biol. Chem. 288, 28524–28534 (2013).
Liang, K. & Vaziri, N. D. Acquired VLDL receptor deficiency in experimental nephrosis. Kidney Int. 51, 1761–1765 (1997).
Zhou, Y. et al. Expression profiling of hepatic genes associated with lipid metabolism in nephrotic rats. Am. J. Physiol. Renal Physiol. 295, F662–F671 (2008).
O'Donnell, M. P. Mechanisms and clinical importance of hypertriglyceridemia in the nephrotic syndrome. Kidney Int. 59, 380–382 (2001).
Kashyap, M. L. et al. Apolipoprotein CII and lipoprotein lipase in human nephrotic syndrome. Atherosclerosis 35, 29–40 (1980).
Ohta, T. & Matsuda, I. Lipid and apolipoprotein levels in patients with nephrotic syndrome. Clin. Chim. Acta 117, 133–143 (1981).
Nasr, S. H. et al. Novel type of renal amyloidosis derived from apolipoprotein-CII. J. Am. Soc. Nephrol. 28, 439–445 (2017).
Tentolouris, N. et al. High postprandial triglyceridemia in patients with type 2 diabetes and microalbuminuria. J. Lipid Res. 48, 218–225 (2007).
Di Bartolo, B., Scherer, D. J., Brown, A., Psaltis, P. J. & Nicholls, S. J. PCSK9 inhibitors in hyperlipidemia: current status and clinical outlook. BioDrugs 31, 167–174 (2017).
Morris, A. W. Nephrotic syndrome: PCSK9: a target for hypercholesterolaemia in nephrotic syndrome. Nat. Rev. Nephrol. 12, 510 (2016).
Pavlakou, P., Liberopoulos, E., Dounousi, E. & Elisaf, M. PCSK9 in chronic kidney disease. Int. Urol. Nephrol. 49, 1015–1024 (2017).
Haas, M. E. et al. The role of proprotein convertase subtilisin/kexin type 9 in nephrotic syndrome-associated hypercholesterolemia. Circulation 134, 61–72 (2016).
Warwick, G. L. et al. Low-density lipoprotein metabolism in the nephrotic syndrome. Metabolism 39, 187–192 (1990).
Vaziri, N. D. & Liang, K. H. Hepatic HMG-CoA reductase gene expression during the course of puromycin-induced nephrosis. Kidney Int. 48, 1979–1985 (1995).
Tsimikas, S. et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N. Engl. J. Med. 353, 46–57 (2005).
Wanner, C. et al. Elevated plasma lipoprotein(a) in patients with the nephrotic syndrome. Ann. Intern. Med. 119, 263–269 (1993).
Glass, C. K. & Witztum, J. L. Atherosclerosis: the road ahead. Cell 104, 503–516 (2001).
Gherardi, E., Rota, E., Calandra, S., Genova, R. & Tamborino, A. Relationship among the concentrations of serum lipoproteins and changes in their chemical composition in patients with untreated nephrotic syndrome. Eur. J. Clin. Invest. 7, 563–570 (1977).
Yvan-Charvet, L., Wang, N. & Tall, A. R. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 30, 139–143 (2010).
Birjmohun, R. S. et al. High-density lipoprotein attenuates inflammation and coagulation response on endotoxin challenge in humans. Arterioscler. Thromb. Vasc. Biol. 27, 1153–1158 (2007).
Murphy, A. J. et al. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler. Thromb. Vasc. Biol. 28, 2071–2077 (2008).
Yuhanna, I. S. et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 7, 853–857 (2001).
Muls, E., Rosseneu, M., Daneels, R., Schurgers, M. & Boelaert, J. Lipoprotein distribution and composition in the human nephrotic syndrome. Atherosclerosis 54, 225–237 (1985).
Vaziri, N. D. HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat. Rev. Nephrol. 12, 37–47 (2016).
Pedigo, C. E. et al. Local TNF causes NFATc1-dependent cholesterol-mediated podocyte injury. J. Clin. Invest. 126, 3336–3350 (2016).
Jao, W., Lewy, P., Norris, S. H., Pollak, V. E. & Pirani, C. L. Lipoid nephrosis: a reassessment. Perspect. Nephrol. Hypertens. 1, 183–198 (1973).
Muso, E. Beneficial effect of LDL-apheresis in refractory nephrotic syndrome. Clin. Exp. Nephrol. 18, 286–290 (2014).
Gordon, T., Castelli, W. P., Hjortland, M. C., Kannel, W. B. & Dawber, T. R. High density lipoprotein as a protective factor against coronary heart disease: the Framingham study. Am. J. Med. 62, 707–714 (1977).
Rye, K. A. & Barter, P. J. Cardioprotective functions of HDLs. J. Lipid Res. 55, 168–179 (2014).
Boden, W. E. et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365, 2255–2267 (2011).
Barter, P. J. et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357, 2109–2122 (2007).
Brunzell, J. D., Zambon, A. & Deeb, S. S. The effect of hepatic lipase on coronary artery disease in humans is influenced by the underlying lipoprotein phenotype. Biochim. Biophys. Acta 1821, 365–372 (2012).
Voight, B. F. et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet 380, 572–580 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01841684 (2015).
Brooks, M. REVEAL: CETP inhibitor anacetrapib meets primary end point. Medscape Nephrology http://www.medscape.com/viewarticle/882173 (2017).
Faraggiana, T. & Churg, J. Renal lipidoses: a review. Hum. Pathol. 18, 661–679 (1987).
Ossoli, A. et al. Lipoprotein X causes renal disease in LCAT deficiency. PLoS ONE 11, e0150083 (2016).
Ferrans, V. J. & Fredrickson, D. S. The pathology of Tangier disease. A light and electron microscopic study. Am. J. Pathol. 78, 101–158 (1975).
Lovric, S. et al. Mutations in sphingosine-1-phosphate lyase cause nephrosis with ichthyosis and adrenal insufficiency. J. Clin. Invest. 127, 912–928 (2017).
Fornoni, A. et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci. Transl Med. 3, 85ra46 (2011).
Choi, H. K. & Seeger, J. D. Glucocorticoid use and serum lipid levels in US adults: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 53, 528–535 (2005).
Leong, K. H., Koh, E. T., Feng, P. H. & Boey, M. L. Lipid profiles in patients with systemic lupus erythematosus. J. Rheumatol 21, 1264–1267 (1994).
MacGregor, A. J. et al. Fasting lipids and anticardiolipin antibodies as risk factors for vascular disease in systemic lupus erythematosus. Ann. Rheum. Dis. 51, 152–155 (1992).
Macfarlane, D. P., Forbes, S. & Walker, B. R. Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. J. Endocrinol. 197, 189–204 (2008).
Vincenti, F., Jensik, S. C., Filo, R. S., Miller, J. & Pirsch, J. A long-term comparison of tacrolimus (FK506) and cyclosporine in kidney transplantation: evidence for improved allograft survival at five years. Transplantation 73, 775–782 (2002).
Mayer, A. D. et al. Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: a report of the European Tacrolimus Multicenter Renal Study Group. Transplantation 64, 436–443 (1997).
Jackson, S. P. & Calkin, A. C. The clot thickens — oxidized lipids and thrombosis. Nat. Med. 13, 1015–1016 (2007).
Podrez, E. A. et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat. Med. 13, 1086–1095 (2007).
Gyebi, L., Soltani, Z. & Reisin, E. Lipid nephrotoxicity: new concept for an old disease. Curr. Hypertens. Rep. 14, 177–181 (2012).
Thomas, M. E., Harris, K. P., Walls, J., Furness, P. N. & Brunskill, N. J. Fatty acids exacerbate tubulointerstitial injury in protein-overload proteinuria. Am. J. Physiol. Renal Physiol. 283, F640–F647 (2002).
Kamijo, A. et al. Urinary free fatty acids bound to albumin aggravate tubulointerstitial damage. Kidney Int. 62, 1628–1637 (2002).
Schermer, B. & Benzing, T. Lipid-protein interactions along the slit diaphragm of podocytes. J. Am. Soc. Nephrol. 20, 473–478 (2009).
Schlondorff, D. Cellular mechanisms of lipid injury in the glomerulus. Am. J. Kidney Dis. 22, 72–82 (1993).
Nishida, Y., Oda, H. & Yorioka, N. Effect of lipoproteins on mesangial cell proliferation. Kidney Int. Suppl. 71, S51–S53 (1999).
Stevenson, F. T., Shearer, G. C. & Atkinson, D. N. Lipoprotein-stimulated mesangial cell proliferation and gene expression are regulated by lipoprotein lipase. Kidney Int. 59, 2062–2068 (2001).
Shearer, G. C. et al. Hypoalbuminemia and proteinuria contribute separately to reduced lipoprotein catabolism in the nephrotic syndrome. Kidney Int. 59, 179–189 (2001).
Zhong, S. et al. Inflammatory stress exacerbated mesangial foam cell formation and renal injury via disrupting cellular cholesterol homeostasis. Inflammation 38, 959–971 (2015).
Kopp, J. B. et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J. Am. Soc. Nephrol. 22, 2129–2137 (2011).
Genovese, G. et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329, 841–845 (2010).
Freedman, B. I. et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J. Am. Soc. Nephrol. 21, 1422–1426 (2010).
Debiec, H. & Ronco, P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N. Engl. J. Med. 364, 689–690 (2011).
Beck, L. H. Jr et al. M-Type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 361, 11–21 (2009).
Fornoni, A., Merscher, S. & Kopp, J. B. Lipid biology of the podocyte — new perspectives offer new opportunities. Nat. Rev. Nephrol. 10, 379–388 (2014).
Chung, J. J. et al. Albumin-associated free fatty acids induce macropinocytosis in podocytes. J. Clin. Invest. 125, 2307–2316 (2015).
Allison, S. J. Free fatty acid-induced macropinocytosis in podocytes. Nat. Rev. Nephrol. 11, 386 (2015).
Agrawal, S., Guess, A. J., Chanley, M. A. & Smoyer, W. E. Albumin-induced podocyte injury and protection are associated with regulation of COX-2. Kidney Int. 86, 1150–1160 (2014).
Sieber, J. et al. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am. J. Physiol. Renal Physiol. 299, F821–F829 (2010).
Kampe, K., Sieber, J., Orellana, J. M., Mundel, P. & Jehle, A. W. Susceptibility of podocytes to palmitic acid is regulated by fatty acid oxidation and inversely depends on acetyl-CoA carboxylases 1 and 2. Am. J. Physiol. Renal Physiol. 306, F401–F409 (2014).
Martinez-Garcia, C. et al. Renal lipotoxicity-associated inflammation and insulin resistance affects actin cytoskeleton organization in podocytes. PLoS ONE 10, e0142291 (2015).
Eddy, A. A. & Michael, A. F. Acute tubulointerstitial nephritis associated with aminonucleoside nephrosis. Kidney Int. 33, 14–23 (1988).
Eddy, A. A., McCulloch, L., Liu, E. & Adams, J. A relationship between proteinuria and acute tubulointerstitial disease in rats with experimental nephrotic syndrome. Am. J. Pathol. 138, 1111–1123 (1991).
Iwai, T. et al. Stearoyl-CoA desaturase-1 protects cells against lipotoxicity-mediated apoptosis in proximal tubular cells. Int. J. Mol. Sci. 17, E1868 (2016).
Li, C. et al. Intrarenal renin-angiotensin system mediates fatty acid-induced ER stress in the kidney. Am. J. Physiol. Renal Physiol. 310, F351–F363 (2016).
Zuo, N., Zheng, X., Liu, H. & Ma, X. Fenofibrate, a PPARα agonist, protect proximal tubular cells from albumin-bound fatty acids induced apoptosis via the activation of NF-kB. Int. J. Clin. Exp. Pathol. 8, 10653–10661 (2015).
Ruggiero, C. et al. Albumin-bound fatty acids but not albumin itself alter redox balance in tubular epithelial cells and induce a peroxide-mediated redox-sensitive apoptosis. Am. J. Physiol. Renal Physiol. 306, F896–F906 (2014).
Sacks, F. M. et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N. Engl. J. Med. 335, 1001–1009 (1996).
Reiner, Z. et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur. Heart J. 32, 1769–1818 (2011).
Bagga, A., Sharma, A. & Srivastava, R. N. Inefficacy of pefloxacin in steroid-responsive nephrotic syndrome. Pediatr. Nephrol. 9, 793–794 (1995).
Keane, W. F. Lipids and the kidney. Kidney Int. 46, 910–920 (1994).
Culleton, B. F. et al. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int. 56, 2214–2219 (1999).
Falk, R. J. in Acute Renal Failure: A Companion to Brenner & Rector's The Kidney (eds Molitoris, B. A. & Finn, W.) (Saunders, 2001).
Kasiske, B. L. Hyperlipidemia in patients with chronic renal disease. Am. J. Kidney Dis. 32, S142–S156 (1998).
Ordonez, J. D., Hiatt, R. A., Killebrew, E. J. & Fireman, B. H. The increased risk of coronary heart disease associated with nephrotic syndrome. Kidney Int. 44, 638–642 (1993).
Suryawanshi, S. P., Das, B. & Patnaik, A. N. Myocardial infarction in children: two interesting cases. Ann. Pediatr. Cardiol. 4, 81–83 (2011).
Silva, J. M. et al. Premature acute myocardial infarction in a child with nephrotic syndrome. Pediatr. Nephrol. 17, 169–172 (2002).
D'Amico, G. et al. Effect of vegetarian soy diet on hyperlipidaemia in nephrotic syndrome. Lancet 339, 1131–1134 (1992).
Gentile, M. G. et al. Treatment of proteinuric patients with a vegetarian soy diet and fish oil. Clin. Nephrol. 40, 315–320 (1993).
Bell, S., Cooney, J., Packard, C. J., Caslake, M. J. & Deighan, C. J. The effect of omega-3 fatty acids on the atherogenic lipoprotein phenotype in patients with nephrotic range proteinuria. Clin. Nephrol. 77, 445–453 (2012).
Hall, A. V. et al. Omega-3 fatty acid supplementation in primary nephrotic syndrome: effects on plasma lipids and coagulopathy. J. Am. Soc. Nephrol. 3, 1321–1329 (1992).
Rabelink, A. J., Hene, R. J., Erkelens, D. W., Joles, J. A. & Koomans, H. A. Effects of simvastatin and cholestyramine on lipoprotein profile in hyperlipidaemia of nephrotic syndrome. Lancet 2, 1335–1338 (1988).
Thomas, M. E. et al. Simvastatin therapy for hypercholesterolemic patients with nephrotic syndrome or significant proteinuria. Kidney Int. 44, 1124–1129 (1993).
M., S. et al. Evaluation of effects of lovastatin on hyercholesterolaemia and renl functions in nephrotic syndrome. Indian Acad. Clin. Med. 5, 143–146 (2004).
Gheith, O. A. et al. Impact of treatment of dyslipidemia on renal function, fat deposits and scarring in patients with persistent nephrotic syndrome. Nephron 91, 612–619 (2002).
Gheith, O., Sheashaa, H., Abdelsalam, M., Shoeir, Z. & Sobh, M. Efficacy and safety of Monascus purpureus Went rice in subjects with secondary hyperlipidemia. Clin. Exp. Nephrol. 12, 189–194 (2008).
Olbricht, C. J., Wanner, C., Thiery, J. & Basten, A. Simvastatin in nephrotic syndrome. Kidney Int. Suppl. 71, S113–S116 (1999).
Kong, X. et al. Lipid-lowering agents for nephrotic syndrome. Cochrane Database Syst. Rev. 12, CD005425 (2013).
Baigent, C. et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 377, 2181–2192 (2011).
Valeri, A., Gelfand, J., Blum, C. & Appel, G. B. Treatment of the hyperlipidemia of the nephrotic syndrome: a controlled trial. Am. J. Kidney Dis. 8, 388–396 (1986).
Groggel, G. C., Cheung, A. K., Ellis-Benigni, K. & Wilson, D. E. Treatment of nephrotic hyperlipoproteinemia with gemfibrozil. Kidney Int. 36, 266–271 (1989).
Buyukcelik, M. et al. The effects of gemfibrozil on hyperlipidemia in children with persistent nephrotic syndrome. Turk. J. Pediatr. 44, 40–44 (2002).
Kamanna, V. S. & Kashyap, M. L. Mechanism of action of niacin. Am. J. Cardiol. 101, 20B–26B (2008).
Phan, B. A., Dayspring, T. D. & Toth, P. P. Ezetimibe therapy: mechanism of action and clinical update. Vasc. Health Risk Manag. 8, 415–427 (2012).
Kastelein, J. J. et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N. Engl. J. Med. 358, 1431–1443 (2008).
Coleman, J. E. & Watson, A. R. Hyperlipidaemia, diet and simvastatin therapy in steroid-resistant nephrotic syndrome of childhood. Pediatr. Nephrol. 10, 171–174 (1996).
Sanjad, S. A., al-Abbad, A. & al-Shorafa, S. Management of hyperlipidemia in children with refractory nephrotic syndrome: the effect of statin therapy. J. Pediatr. 130, 470–474 (1997).
Hattori, M. et al. A combined low-density lipoprotein apheresis and prednisone therapy for steroid-resistant primary focal segmental glomerulosclerosis in children. Am. J. Kidney Dis. 42, 1121–1130 (2003).
Muso, E. et al. Low density lipoprotein apheresis therapy for steroid-resistant nephrotic syndrome. Kansai-FGS-Apheresis Treatment (K-FLAT) Study Group. Kidney Int. Suppl. 71, S122–S125 (1999).
Muso, E. et al. Immediate therapeutic efficacy of low-density lipoprotein apheresis for drug-resistant nephrotic syndrome: evidence from the short-term results from the POLARIS Study. Clin. Exp. Nephrol. 19, 379–386 (2015).
Muso, E. et al. A prospective observational survey on the long-term effect of LDL apheresis on drug-resistant nephrotic syndrome. Nephron Extra 5, 58–66 (2015).
Suzuki, H., Tsukamoto, T. & Muso, E. Rituximab-resistant nephrotic syndrome with successful induction of remission by low-density lipoprotein apheresis. Ther. Apher. Dial. 21, 295–296 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02314442(2015).
Fitzgerald, K. et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N. Engl. J. Med. 376, 41–51 (2017).
Liu, S. & Vaziri, N. D. Role of PCSK9 and IDOL in the pathogenesis of acquired LDL receptor deficiency and hypercholesterolemia in nephrotic syndrome. Nephrol. Dial. Transplant. 29, 538–543 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03004001 (2017).
Awanami, Y. et al. Successful treatment of a patient with refractory nephrotic syndrome with PCSK9 inhibitors: a case report. BMC Nephrol. 18, 221 (2017).
Kazi, D. S. et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA 316, 743–753 (2016).
Vaziri, N. D. & Liang, K. H. Acyl-coenzyme A:cholesterol acyltransferase inhibition ameliorates proteinuria, hyperlipidemia, lecithin-cholesterol acyltransferase, SRB-1, and low-denisty lipoprotein receptor deficiencies in nephrotic syndrome. Circulation 110, 419–425 (2004).
Tardif, J. C. et al. Effects of the acyl coenzyme A: cholesterol acyltransferase inhibitor avasimibe on human atherosclerotic lesions. Circulation 110, 3372–3377 (2004).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02235857 (2016).
[No authors listed.] Chapter 2: General principles in the management of glomerular disease. Kidney Int. Suppl. 2, 156–162 (2012).
Lechner, B. L., Bockenhauer, D., Iragorri, S., Kennedy, T. L. & Siegel, N. J. The risk of cardiovascular disease in adults who have had childhood nephrotic syndrome. Pediatr. Nephrol. 19, 744–748 (2004).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00004466 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01845428 (2017).
Acknowledgements
The authors acknowledge the expert assistance of L. Feurer (Center for Clinical and Translational Research, The Research Institute at Nationwide Childrens Hospital, Columbus, Ohio,USA) in creating initial drafts of the figures in this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for the article, and writing, reviewing and editing the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Sialylation
-
Addition of sialic acid groups onto molecules such as oligosaccharides and carbohydrates.
- Glycocalyx
-
Layer of glycoproteins and sugar moieties surrounding the outer surface of the cell membrane of some bacteria, epithelia and other cells.
- Tangier disease
-
A rare inherited disorder characterized by significantly reduced levels of HDL in the blood.
- Lipid raft
-
A subdomain of the plasma membrane that contain high concentrations of cholesterol and glycosphingolipids.
- Lipid apheresis
-
A non-surgical therapy and a form of apheresis that removes LDL from a patient's blood.
Rights and permissions
About this article
Cite this article
Agrawal, S., Zaritsky, J., Fornoni, A. et al. Dyslipidaemia in nephrotic syndrome: mechanisms and treatment. Nat Rev Nephrol 14, 57–70 (2018). https://doi.org/10.1038/nrneph.2017.155
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2017.155
This article is cited by
-
Longitudinal analysis of blood pressure and lipids in childhood nephrotic syndrome
Pediatric Nephrology (2024)
-
Injection of an improperly stored proprotein convertase subtilisin/kexin type 9 monoclonal antibody in a patient with secondary dyslipidemia from nephrotic syndrome: a case report
Journal of Medical Case Reports (2023)
-
B cell-derived anti-beta 2 glycoprotein I antibody mediates hyperhomocysteinemia-aggravated hypertensive glomerular lesions by triggering ferroptosis
Signal Transduction and Targeted Therapy (2023)
-
Long-term visit-to-visit variability in low-density lipoprotein cholesterol is associated with poor cardiovascular and kidney outcomes in patients with primary nephrotic syndrome
International Urology and Nephrology (2023)
-
Research progress of nephrotic syndrome accompanied by thromboembolism
International Urology and Nephrology (2023)