Abstract
Interferon β and glatiramer acetate have been available for the treatment of multiple sclerosis (MS) for over a decade and their efficacy and safety are well established. These agents have detectable effects on the immune system, but have not been associated with a breakdown of immune surveillance. Novel MS therapies have been approved, or are awaiting approval, that differ from established immunotherapies with regard to their mechanisms of action, modes of administration, adverse-effect profiles and, possibly, the clinical and paraclinical benefits that they may provide for patients. Neurologists will soon be required to make complex treatment decisions with their patients on the basis of very limited clinical data and evidence. Under these circumstances, optimal assessment of risks and benefits will be challenging. In this Review, the anticipated benefits of novel therapies, including reduction in disease activity, possible prevention of disability, and improvement in quality of life, are outlined. In addition, the current acceptance of potential risks—including serious or even life-threatening adverse effects, the likelihood of which may rise with increased cumulative exposure to a particular agent—by patients with MS will be reviewed.
Key Points
-
Emergence of new pharmacotherapies for multiple sclerosis (MS) poses numerous advantages and challenges to the clinical neurologist
-
Decisions on the most appropriate agent for MS patients at any time during the relapsing disease course is becoming more complex
-
The balance between benefit and risk may be perceived differently between individual patients owing to their personal expectations and apprehensions as well as cultural differences
-
Assessments of available therapies should not be static, but should be conducted longitudinally, as both risks and benefits may change over time
-
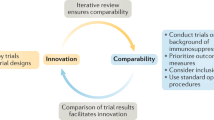
Head-to-head comparative trials conducted using identical methodology in patients randomized from the same source population are required to compare the efficacy of different MS treatments
-
The identification of MS biomarkers for disease activity will be paramount for any rational treatment choices
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ann Marrie, R. & Rudick, R. A. Drug Insight: interferon treatment in multiple sclerosis. Nat. Clin. Pract. Neurol. 2, 34–44 (2006).
Zvartau-Hind, M. et al. Glatiramer acetate for multiple sclerosis: a comprehensive review of mechanisms and clinical efficacy. Expert Rev. Neurother. 2, 285–294 (2002).
Kingwell, E. et al. Cardiotoxicity and other adverse events associated with mitoxantrone treatment for MS. Neurology 74, 1822–1826 (2010).
Ransohoff, R. M. Natalizumab for multiple sclerosis. N. Engl. J. Med. 356, 2622–2629 (2007).
Linker, R. A., Kieseier, B. C. & Gold, R. Identification and development of new therapeutics for multiple sclerosis. Trends Pharmacol. Sci. 29, 558–565 (2008).
Hemmer, B., Stüve, O., Kieseier, B., Schellekens, H. & Hartung, H. P. Immune response to immunotherapy: the role of neutralising antibodies to interferon beta in the treatment of multiple sclerosis. Lancet Neurol. 4, 403–412 (2005).
Gorelik, L. et al. Anti-JC virus antibodies: implications for PML risk stratification. Ann. Neurol. 68, 295–303 (2010).
[No authors listed] JCV Antibody Program (STRATIFY-2) ClinicalTrials.gov [online], (2010).
Heesen, C. et al. Risk perception in natalizumab-treated multiple sclerosis patients and their neurologists. Mult. Scler. 16, 1507–1512 (2010).
Clifford, D. B. et al. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 9, 438–446 (2010).
Rothwell, P. M., McDowell, Z., Wong, C. K. & Dorman, P. J. Doctors and patients don't agree: cross sectional study of patients' and doctors' perceptions and assessments of disability in multiple sclerosis. BMJ 314, 1580–1583 (1997).
Nortvedt, M. W., Riise, T., Myhr, K. M. & Nyland, H. I. Quality of life in multiple sclerosis: measuring the disease effects more broadly. Neurology 53, 1098–1103 (1999).
Zwibel, H. Health and quality of life in patients with relapsing multiple sclerosis: making the intangible tangible. J. Neurol. Sci. 287 (Suppl. 1), S11–S16 (2009).
Forbes, A., While, A., Mathes, L. & Griffiths, P. Health problems and health-related quality of life in people with multiple sclerosis. Clin. Rehabil. 20, 67–78 (2006).
Miller, D. M. & Allen, R. Quality of life in multiple sclerosis: determinants, measurement, and use in clinical practice. Curr. Neurol. Neurosci. Rep. 10, 397–406 (2010).
Fisk, J. D., Pontefract, A., Ritvo, P. G., Archibald, C. J. & Murray, T. J. The impact of fatigue on patients with multiple sclerosis. Can. J. Neurol. Sci. 21, 9–14 (1994).
Fruehwald, S., Loeffler-Stastka, H., Eher, R., Saletu, B. & Baumhackl, U. Depression and quality of life in multiple sclerosis. Acta Neurol. Scand. 104, 257–261 (2001).
Chiaravalloti, N. D. & DeLuca, J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 7, 1139–1151 (2008).
Rudick, R. A. & Miller, D. M. Health-related quality of life in multiple sclerosis: current evidence, measurement and effects of disease severity and treatment. CNS Drugs 22, 827–839 (2008).
Spain, L. A., Tubridy, N., Kilpatrick, T. J., Adams, S. J. & Holmes, A. C. Illness perception and health-related quality of life in multiple sclerosis. Acta Neurol. Scand. 116, 293–299 (2007).
Jacobs, L. D. et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann. Neurol. 39, 285–294 (1996).
Johnson, K. P. Copolymer 1 reduces relapse rate and improves disability in relapsing–remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 45, 1268–1276 (1995).
[No authors listed] Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 352, 1498–1504 (1998).
[No authors listed] Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. The IFNβ Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group. Neurology 45, 1277–1285 (1995).
Polman, C. H. et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 354, 899–910 (2006).
Havrdova, E. et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing–Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol. 8, 254–260 (2009).
Jones, J. L. et al. Improvement in disability after alemtuzumab treatment of multiple sclerosis is associated with neuroprotective autoimmunity. Brain 133, 2232–2247 (2010).
Munschauer, F. et al. Natalizumab significantly increases the cumulative probability of sustained improvement in physical disability. Mult. Scler. 14 (Suppl. 1), S167–S168 (2008).
Giovannoni, G. et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N. Engl. J. Med. 362, 416–426 (2010).
Kappos, L. et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 362, 387–401 (2010).
[No authors listed] Interferon beta-1b is effective in relapsing–remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNβ Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology 43, 655–661 (1993).
Klawiter, E. C., Cross, A. H. & Naismith, R. T. The present efficacy of multiple sclerosis therapeutics: is the new 66% just the old 33%? Neurology 73, 984–990 (2009).
Mikol, D. D. et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol. 7, 903–914 (2008).
Panitch, H. et al. Randomized, comparative study of interferon beta-1a treatment regimens in MS: The EVIDENCE Trial. Neurology 59, 1496–1506 (2002).
Sormani, M. P. et al. Will Rogers phenomenon in multiple sclerosis. Ann. Neurol. 64, 428–433 (2008).
Koch-Henriksen, N. & Sørensen, P. S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 9, 520–532 (2010).
Cree, B. A., Al-Sabbagh, A., Bennett, R. & Goodin, D. Response to interferon beta-1a treatment in African American multiple sclerosis patients. Arch. Neurol. 62, 1681–1683 (2005).
Cree, B. A. et al. Modification of multiple sclerosis phenotypes by African ancestry at HLA. Arch. Neurol. 66, 226–233 (2009).
Mikol, D. et al. The REGARD trial: a randomised assessor-blinded trial comparing interferon beta-la and glatiramer acetate in relapsing–remitting multiple sclerosis. Mult. Scler. 13 (Suppl. 2), S269 (2007).
O'Connor, P. et al. 250 μg or 500 μg interferon beta-1b versus 20 mg glatiramer acetate in relapsing–remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol. 8, 889–897 (2009).
Cohen, J. A. et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 362, 402–415 (2010).
Coles, A. J. et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N. Engl. J. Med. 359, 1786–1801 (2008).
Ghalie, R. G. et al. Cardiac adverse effects associated with mitoxantrone (novantrone) therapy in patients with MS. Neurology 59, 909–913 (2002).
Ellis, R. & Boggild, M. Therapy-related acute leukaemia with mitoxantrone: what is the risk and can we minimise it? Mult. Scler. 15, 505–508 (2009).
Suntharalingam, G. et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 355, 1018–1028 (2006).
Giezen, T. J. et al. Safety-related regulatory actions for biologicals approved in the United States and the European Union. JAMA 300, 1887–1896 (2008).
Bozic, C. et al. Utilization and safety of natalizumab in patients with relapsing multiple sclerosis. Mult. Scler. 16 (Suppl.1), S315 (2010).
European Union. European Medicines Agency [online], (2010).
Ramtahal, J., Jacob, A., Das, K. & Boggild, M. Sequential maintenance treatment with glatiramer acetate after mitoxantrone is safe and can limit exposure to immunosuppression in very active, relapsing remitting multiple sclerosis. J. Neurol. 253, 1160–1164 (2006).
Vollmer, T. et al. Glatiramer acetate after induction therapy with mitoxantrone in relapsing multiple sclerosis. Mult. Scler. 14, 663–670 (2008).
Boggild, M. Immunosuppression followed by immunomodulation. J. Neurol. Sci. 277 (Suppl. 1), S50–S54 (2009).
Le Page, E. & Edan, G. Long-term experience with induction treatment regimens in multiple sclerosis. J. Neurol. Sci. 277, S46–S49 (2009).
Dörr, J. et al. Severe cardiac failure in a patient with multiple sclerosis following low-dose mitoxantrone treatment. Neurology. 73, 991–993 (2009).
Cotte, S. et al. ABC-transporter gene-polymorphisms are potential pharmacogenetic markers for mitoxantrone response in multiple sclerosis. Brain 132. 2517–2530 (2009).
Stüve, O. et al. Immunologic, clinical, and radiologic status 14 months after cessation of natalizumab therapy. Neurology 72, 396–401 (2009).
Rudick, R. A. et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N. Engl. J. Med. 354, 911–923 (2006).
Killestein, J. et al. Natalizumab drug holiday in multiple sclerosis: poorly tolerated. Ann. Neurol. 68, 392–395 (2010).
West, T. W. & Cree, B. A. Natalizumab dosage suspension: are we helping or hurting? Ann. Neurol. 68, 395–399 (2010).
Berger, J. R. et al. Considerations on discontinuing natalizumab for the treatment of multiple sclerosis. Ann. Neurol. 68, 409–411 (2010).
[No listed authors] Treatment interruption of natalizumab (RESTORE) ClinialTrials.gov [online] (2010).
Kieseier, B. C., Wiendl, H., Hartung, H. P. & Stüve, O. The future of multiple sclerosis therapy. Pharmacol. Res. 60, 207–211 (2009).
Author information
Authors and Affiliations
Contributions
B. C. Kieseier and O. Stüve contributed equally to researching data, discussion of content, writing and reviewing, and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
B. C. Kieseier has acted as a consultant and been a member of a speakers bureau (received honoraria) from Bayer–Schering Pharma, Biogen Idec, Merck Serono, Novartis, Teva Neuroscience. O. Stüve has acted as a consultant for EMO Serono, Novartis, Roche and Teva Neuroscience.
Rights and permissions
About this article
Cite this article
Kieseier, B., Stüve, O. A critical appraisal of treatment decisions in multiple sclerosis—old versus new. Nat Rev Neurol 7, 255–262 (2011). https://doi.org/10.1038/nrneurol.2011.41
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2011.41
This article is cited by
-
Mesenchymal Stem Cells Ameliorate Cuprizone-Induced Demyelination by Targeting Oxidative Stress and Mitochondrial Dysfunction
Cellular and Molecular Neurobiology (2021)
-
The TOTEM RRMS (Testosterone Treatment on neuroprotection and Myelin Repair in Relapsing Remitting Multiple Sclerosis) trial: study protocol for a randomized, double-blind, placebo-controlled trial
Trials (2020)
-
Astrocyte ablation induced by La-aminoadipate (L-AAA) potentiates remyelination in a cuprizone demyelinating mouse model
Metabolic Brain Disease (2019)
-
Immune-mediated neuropathies
Nature Reviews Disease Primers (2018)
-
Optimizing treatment success in multiple sclerosis
Journal of Neurology (2016)