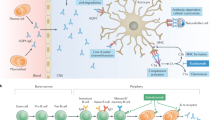
Abstract
Terrible, agonizing, wretched, sickening and unbearable—these are words frequently used by patients with neuromyelitis optica (NMO) to describe a very common symptom of their disease: pain. More than 80% of patients with NMO experience pain from this condition, which severely affects their quality of life. At present, there is no known therapy that produces satisfactory relief from NMO-associated pain. In fact, contemporary pain therapy is largely ineffective in these patients, suggesting that the mechanisms underlying pain in NMO differ substantially from those of other treatable causes of pain. Until now, the near-complete neglect of research into pain mechanisms in NMO has precluded rational pain therapy. In this Perspectives article, expertise from the fields of neuroimmunology, neurology and pain research is combined to explore, for the first time, the mechanisms underlying pain in patients with NMO, and to identify molecular and cellular targets for therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wingerchuk, D. M., Lennon, V. A., Lucchinetti, C. F., Pittock, S. J. & Weinshenker, B. G. The spectrum of neuromyelitis optica. Lancet Neurol. 6, 805–815 (2007).
Papadopoulos, M. C. & Verkman, A. S. Aquaporin water channels in the nervous system. Nat. Rev. Neurosci. 14, 265–277 (2013).
Lennon, V. A. et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364, 2106–2112 (2004).
Jarius, S. & Wildemann, B. Aquaporin-4 antibodies (NMO-IgG) as a serological marker of neuromyelitis optica: a critical review of the literature. Brain Pathol. 23, 661–683 (2013).
Bradl, M. et al. Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann. Neurol. 66, 630–643 (2009).
Bennett, J. L. et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann. Neurol. 66, 617–629 (2009).
Kinoshita, M. et al. Neuromyelitis optica: passive transfer to rats by human immunoglobulin. Biochem. Biophys. Res. Commun. 386, 623–627 (2009).
Saadoun, S. et al. Intra-cerebral injection of neuromyelitis optica imunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain 133, 349–361 (2010).
Bradl, M. & Lassmann, H. Experimental models of neuromyelitis optica. Brain Pathol. 24, 74–82 (2014).
Sato, D. K., Lana-Peixoto, M. A., Fujihara, K. & de Seze, J. Clinical spectrum and treatment of neuromyelitis optica spectrum disorders: evolution and current status. Brain Pathol. 23, 647–660 (2013).
Kanamori, Y. et al. Pain in neuromyelitis optica and its effect on quality of life: a cross-sectional study. Neurology 77, 652–658 (2011).
Qian, P. et al. Association of neuromyelitis optica with severe and intractable pain. Arch. Neurol. 69, 1482–1487 (2012).
Zhao, S., Mutch, K., Elsone, L., Nurmikko, T. & Jacob, A. Neuropathic pain in neuromyelitis optica affects activities of daily living and quality of life. Mult. Scler. http://dx.doi.org/10.1177/1352458514522103.
Pellkofer, H. L. et al. The major brain endocannabinoid 2-AG controls neuropathic pain and mechanical hyperalgesia in patients with neuromyelitis optica. PLoS ONE 8, e71500 (2013).
Kim, S. M., Go, M. J., Sung, J. J., Park, K. S. & Lee, K. W. Painful tonic spasm in neuromyelitis optica: incidence, diagnostic utility, and clinical characteristics. Arch. Neurol. 69, 1026–1031 (2012).
Evangelopoulos, M. E. et al. Neuromyelitis optica spectrum disease with positive autoimmune indices: a case report and review of the literature. Case Rep. Med. 2011, 393568 (2011).
Elsone, L. et al. Neuropathic pruritus (itch) in neuromyelitis optica. Mult. Scler. 19, 475–479 (2013).
Milligan, E. D. & Watkins, L. R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 10, 23–36 (2009).
Marchand, F., Perretti, M. & McMahon, S. B. Role of the immune system in chronic pain. Nat. Rev. Neurosci. 6, 521–532 (2005).
Xanthos, D. N. & Sandkühler, J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat. Rev. Neurosci. 15, 43–53 (2014).
Basbaum, A. I., Bautista, D. M., Scherrer, G. & Julius, D. Cellular and molecular mechanisms of pain. Cell 139, 267–284 (2009).
Lu, Y. & Perl, E. R. Selective action of noradrenaline and serotonin on neurones of the spinal superficial dorsal horn in the rat. J. Physiol. 582, 127–136 (2007).
Sandkühler, J. Models and mechanisms of hyperalgesia and allodynia. Physiol. Rev. 89, 707–758 (2009).
Lucchinetti, C. F. et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain 125, 1450–1461 (2002).
Misu, T. et al. Presence of six different lesion types suggests diverse mechanisms of tissue injury in the lesions of neuromyelitis optica. Acta Neuropathol. 125, 815–827 (2013).
Lucchinetti, C. F. et al. The pathology of an autoimmune astrocytopathy: lessons learned from neuromyelitis optica. Brain Pathol. 24, 83–97 (2014).
Jarius, S. et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J. Neuroinflammation 9, 14 (2012).
Wingerchuk, D. M., Hogancamp, W. F., O'Brien, P. C. & Weinshenker, B. G. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology 53, 1107–1114 (1999).
Collongues, N. et al. Neuromyelitis optica in France: a multicenter study of 125 patients. Neurology 74, 736–742 (2010).
Wingerchuk, D. M., Lennon, V. A., Pittock, S. J., Lucchinetti, C. F. & Weinshenker, B. G. Revised diagnostic criteria for neuromyelitis optica. Neurology 66, 1485–1489 (2006).
Nakamura, M. et al. Preferential spinal central gray matter involvement in neuromyelitis optica. An MRI study. J. Neurol. 255, 163–170 (2008).
Roemer, S. F. et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 130, 1194–1205 (2007).
Misu, T., Fujihara, K., Nakashima, I., Sato, S. & Itoyama, Y. Intractable hiccup and nausea with periaqueductal lesions in neuromyelitis optica. Neurology 65, 1479–1482 (2005).
Hinson, S. R. et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology 69, 2221–2231 (2007).
Rossi, A., Ratelade, J., Papadopoulos, M. C., Bennett, J. L. & Verkman, A. S. Neuromyelitis optica IgG does not alter aquaporin-4 water permeability, plasma membrane M1/M23 isoform content, or supramolecular assembly. Glia 60, 2027–2039 (2012).
Hinson, S. R. et al. Aquaporin-4-binding autoantibodies in patients with neuromyelitis optica impair glutamate transport by down-regulating EAAT2. J. Exp. Med. 205, 2473–2481 (2008).
Marignier, R. et al. Oligodendrocytes are damaged by neuromyelitis optica immunoglobulin G via astrocyte injury. Brain 133, 2578–2591 (2010).
Matsuoka, T., Suzuki, S. O., Suenaga, T., Iwaki, T. & Kira, J. Reappraisal of aquaporin-4 astrocytopathy in Asian neuromyelitis optica and multiple sclerosis patients. Brain Pathol. 21, 516–532 (2011).
Matsushita, T. et al. Astrocytopathy in neuromyelitis optica, multiple sclerosis and Balo's disease [Japanese]. Rinsho Shinkeigaku 51, 898–900 (2011).
Sharma, R. et al. Inflammation induced by innate immunity in the central nervous system leads to primary astrocyte dysfunction followed by demyelination. Acta Neuropathol. 120, 223–236 (2010).
Parratt, J. D. & Prineas, J. W. Neuromyelitis optica: a demyelinating disease characterized by acute destruction and regeneration of perivascular astrocytes. Mult. Scler. 16, 1156–1172 (2010).
Nishiyama, S. et al. A case of NMO seropositive for aquaporin-4 antibody more than 10 years before onset. Neurology 72, 1960–1961 (2009).
Uzawa, A., Masahiro, M. & Kuwabara, S. Cytokines and chemokines in neuromyelitis optica: pathogenetic and therapeutic implications. Brain Pathol. 24, 67–73 (2014).
Olechowski, C. J., Truong, J. J. & Kerr, B. J. Neuropathic pain behaviours in a chronic-relapsing model of experimental autoimmune encephalomyelitis (EAE). Pain 141, 156–164 (2009).
Gruber-Schoffnegger, D. et al. Induction of thermal hyperalgesia and synaptic long-term potentiation in the spinal cord lamina I by TNF-α and IL-1β is mediated by glial cells. J. Neurosci. 33, 6540–6551 (2013).
Park, C. K. et al. Resolving TRPV1- and TNF-α-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J. Neurosci. 31, 15072–15085 (2011).
Ikeda, H. et al. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science 312, 1659–1662 (2006).
Drdla, R., Gassner, M., Gingl, E. & Sandkühler, J. Induction of synaptic long-term potentiation after opioid withdrawal. Science 325, 207–210 (2009).
Meng, X. et al. Spinal interleukin-17 promotes thermal hyperalgesia and NMDA NR1 phosphorylation in an inflammatory pain rat model. Pain 154, 294–305 (2013).
Nakatsuka, T., Tsuzuki, K., Ling, J. X., Sonobe, H. & Gu, J. G. Distinct roles of P2X receptors in modulating glutamate release at different primary sensory synapses in rat spinal cord. J. Neurophysiol. 89, 3243–3252 (2003).
Hansen, R. R. & Malcangio, M. Astrocytes—multitaskers in chronic pain. Eur. J. Pharmacol. 716, 120–128 (2013).
Donnelly-Roberts, D., McGaraughty, S., Shieh, C. C., Honore, P. & Jarvis, M. F. Painful purinergic receptors. J. Pharmacol. Exp. Ther. 324, 409–415 (2008).
Jarius, S. & Wildemann, B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nat. Rev. Neurol. 6, 383–392 (2010).
Moga, D., Hof, P. R., Vissavajjhala, P., Moran, T. M. & Morrison, J. H. Parvalbumin-containing interneurons in rat hippocampus have an AMPA receptor profile suggestive of vulnerability to excitotoxicity. J. Chem. Neuroanat. 23, 249–253 (2002).
Zeilhofer, H. U., Wildner, H. & Yevenes, G. E. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol. Rev. 92, 193–235 (2012).
Coull, J. A. et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021 (2005).
Susser, E., Sprecher, E. & Yarnitsky, D. Paradoxical heat sensation in healthy subjects: peripherally conducted by Aδ or C fibres? Brain 122, 239–246 (1999).
Sigel, E. et al. The major central endocannabinoid directly acts at GABAA receptors. Proc. Natl Acad. Sci. USA 108, 18150–18155 (2011).
Kallendrusch, S. et al. Intrinsic up-regulation of 2-AG favors an area specific neuronal survival in different in vitro models of neuronal damage. PLoS ONE 7, e51208 (2012).
Martinez-Hernandez, A., Bell, K. P. & Norenberg, M. D. Glutamine synthetase: glial localization in brain. Science 195, 1356–1358 (1977).
Albrecht, J., Sidoryk-Wegrzynowicz, M., Zielinska, M. & Aschner, M. Roles of glutamine in neurotransmission. Neuron Glia Biol. 6, 263–276 (2010).
Behbehani, M. M. Functional characteristics of the midbrain periaqueductal gray. Prog. Neurobiol. 46, 575–605 (1995).
Kremer, L. et al. Brainstem manifestations in neuromyelitis optica: a multicenter study of 258 patients. Mult. Scler. http://dx.doi.org/10.1177/1352458513507822.
Verkman, A. S., Phuan P.-W., Asavapanumas, N. & Tradtrantip, L. Biology of AQP4 and anti-AQP4 antibody: therapeutic implications for NMO. Brain Pathol. 23, 684–695 (2013).
Kumar, A. & Loane, D. J. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav. Immun. 26, 1191–1201 (2012).
Wang, X. et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc. Natl Acad. Sci. USA 109, 6325–6330 (2012).
Wallace, M. S., Lam, V. & Schettler, J. NGX426, an oral AMPA-kainate antagonist, is effective in human capsaicin-induced pain and hyperalgesia. Pain Med. 13, 1601–1610 (2012).
Volpi, C., Fazio, F. & Fallarino, F. Targeting metabotropic glutamate receptors in neuroimmune communication. Neuropharmacology 63, 501–506 (2012).
Sandkühler, J. & Lee, J. How to erase memory traces of pain and fear. Trends Neurosci. 36, 343–352 (2013).
Drdla-Schutting, R., Benrath, J., Wunderbaldinger, G. & Sandkühler, J. Erasure of a spinal memory trace of pain by a brief, high-dose opioid administration. Science 335, 235–238 (2012).
Taira, T. et al. A new approach to control central deafferentation pain: spinal intrathecal baclofen. Stereotact. Funct. Neurosurg. 65, 101–105 (1995).
Dykstra, D., Stuckey, M., DesLauriers, L., Chappuis, D. & Krach, L. Intrathecal baclofen in the treatment of spasticity. Acta Neurochir. Suppl. 97, 163–171 (2007).
Rog, D. J., Nurmikko, T. J., Friede, T. & Young, C. A. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 27, 812–819 (2005).
Hunt, S. P. & Mantyh, P. W. The molecular dynamics of pain control. Nat. Rev. Neurosci. 2, 83–91 (2001).
Pittock, S. J. et al. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch. Neurol. 63, 964–968 (2006).
Kitic, M. et al. Intrastriatal injection of interleukin 1 β triggers the formation of neuromyelitis optica-like lesions in NMO-IgG seropositive rats. Acta Neuropathol. Commun. 1, 5 (2013).
Uzawa, A. et al. Cytokine and chemokine profiles in neuromyelitis optica: significance of interleukin-6. Mult. Scler. 16, 1443–1452 (2010).
Uzawa, A. et al. Markedly increased CSF interleukin-6 levels in neuromyelitis optica, but not in multiple sclerosis. J. Neurol. 256, 2082–2084 (2009).
Icoz, S. et al. Enhanced IL-6 production in aquaporin-4 antibody positive neuromyelitis optica patients. Int. J. Neurosci. 120, 71–75 (2010).
Wei, X. H. et al. The up-regulation of IL-6 in DRG and spinal dorsal horn contributes to neuropathic pain following L5 ventral root transection. Exp. Neurol. 241, 159–168 (2013).
Guptarak, J. et al. Inhibition of IL-6 signaling: a novel therapeutic approach to treating spinal cord injury pain. Pain 154, 1115–1128 (2013).
DeLeo, J. A., Colburn, R. W., Nichols, M. & Malhotra, A. Interleukin-6-mediated hyperalgesia/allodynia and increased spinal IL-6 expression in a rat mononeuropathy model. J. Interferon Cytokine Res. 16, 695–700 (1996).
Arruda, J. L., Sweitzer, S., Rutkowski, M. D. & DeLeo, J. A. Intrathecal anti-IL-6 antibody and IgG attenuates peripheral nerve injury-induced mechanical allodynia in the rat: possible immune modulation in neuropathic pain. Brain Res. 879, 216–225 (2000).
Araki, M. et al. Clinical improvement in a patient with neuromyelitis optica following therapy with the anti-IL-6 receptor monoclonal antibody tocilizumab. Mod. Rheumatol. 23, 827–831 (2013).
Ishizu, T. et al. Intrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosis. Brain 128, 988–1002 (2005).
Wang, H. et al. Interleukin 17 gene polymorphism is associated with anti-aquaporin 4 antibody-positive neuromyelitis optica in the Southern Han Chinese—a case control study. J. Neurol. Sci. 314, 26–28 (2012).
Wang, H. H. et al. Interleukin-17-secreting T cells in neuromyelitis optica and multiple sclerosis during relapse. J. Clin. Neurosci. 18, 1313–1317 (2011).
Wang, K. C. et al. Elevated plasma high-mobility group box 1 protein is a potential marker for neuromyelitis optica. Neuroscience 226, 510–516 (2012).
Ren, P. C. et al. High-mobility group box 1 contributes to mechanical allodynia and spinal astrocytic activation in a mouse model of type 2 diabetes. Brain Res. Bull. 88, 332–337 (2012).
Acknowledgements
We thank Takashi Yamamura for being the first to call our attention to the connection between neuromyelitis optica and pain. Our work is supported by the Austrian Science Fund (grant numbers P25240-B24 to M.B., I916-B13 [International Programme, Eugène Devic European Network] to H.L. and P22306-B19 to J.S.), and in part by the Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Technology and the Ministry of Health, Labor and Welfare of Japan to K.F.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, and contributed to discussion of content and review/editing of the manuscript before submission. M.B., H.L. and J.S. wrote the article.
Corresponding author
Ethics declarations
Competing interests
M.B. has received travel support from Mitsubishi Tanabe. H.L. is a consultant for Amgen, Biogen and Baxter Healthcare and has received speaker honoraria from Novartis, Biogen, Merck Serono and TEVA. I.N. has received travel funding and speaker honoraria from Bayer Yakuhin, Biogen Idec Japan, Mitsubishi Tanabe and Novartis, and research funding from Mitsubishi Chemical Medience. T.M. has received speaker honoraria from Bayer Schering, Biogen Idec Japan, Mitsubishi Tanabe, Asahi Kasei Medical and Astellas, and research support from Bayer Schering, Biogen Idec Japan, Asahi Kasei Kuraray Medical, the Chemo-Sero-Therapeutic Research Institute, TEVA, Mitsubishi Tanabe and Teijin. K.F. is on the scientific advisory boards for Bayer Schering, Biogen Idec, Mitsubishi Tanabe, Novartis, Chugai, Ono, Nihon, Merck Serono and Alexion; he has received travel funding and speaker honoraria from Bayer Schering, Biogen Idec, Eisai, Mitsubishi Tanabe, Novartis, Astellas, Takeda, Asahi Kasei Medical and Daiichi Sankyo; and has received research support from Bayer Schering, Biogen Idec Japan, Asahi Kasei Medical, the Chemo-Sero-Therapeutic Research Institute, TEVA, Mitsubishi Tanabe, Teijin, Eisai and Kowa. J.S. has received speaker honoraria from: Astra Zeneca, CT Arzneimittel, DR. KADE, Eli Lilly, Grünenthal, Janssen-Cilag, Mundipharma, Pfizer, TEVA. He is also a member of the scientific advisory board for Grünenthal. Y.K. declares no competing interests.
Rights and permissions
About this article
Cite this article
Bradl, M., Kanamori, Y., Nakashima, I. et al. Pain in neuromyelitis optica—prevalence, pathogenesis and therapy. Nat Rev Neurol 10, 529–536 (2014). https://doi.org/10.1038/nrneurol.2014.129
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2014.129
This article is cited by
-
Central neuropathic pain
Nature Reviews Disease Primers (2023)
-
Cerebellar connectome alterations and associated genetic signatures in multiple sclerosis and neuromyelitis optica spectrum disorder
Journal of Translational Medicine (2023)
-
Update on the diagnosis and treatment of neuromyelits optica spectrum disorders (NMOSD) – revised recommendations of the Neuromyelitis Optica Study Group (NEMOS). Part I: Diagnosis and differential diagnosis
Journal of Neurology (2023)
-
The risk factors of neuropathic pain in neuromyelitis optica spectrum disorder: a retrospective case-cohort study
BMC Neurology (2022)
-
Sexual Dysfunction in Women with Neuromyelitis Optica Spectrum Disorders and Multiple Sclerosis
Sexuality and Disability (2022)