Key Points
-
The misfolding of proteins and their subsequent deposition as amyloid in peripheral nerves lead to sensory and autonomic neuropathy
-
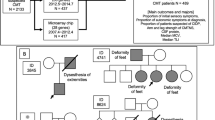
A new hereditary form of neuropathy, prion protein (PrP) systemic amyloidosis, is caused by truncation mutations of the prion protein gene PRNP
-
Some acquired prion diseases are known to have a peripheral phase in their pathogenesis, but were not previously thought to cause peripheral disease
-
The new prion disease is unlikely to pose any risk of iatrogenic transmission, but precautionary measures are recommended
-
Peripheral manifestations are increasingly recognized in common neurodegenerative diseases associated with protein misfolding
-
Regional variability in protein deposition and toxicity associated with protein-misfolding disorders is only partly explained by known factors, and is a key area for future research
Abstract
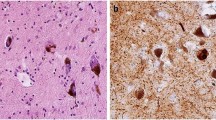
Prion diseases are typically recognized as rapidly progressive dementing illnesses that also feature myoclonus and cerebellar ataxia. Several families have now been described with a late-onset hereditary sensory and autonomic neuropathy caused by truncation of prion protein (PrP), and associated with systemic amyloidosis, which was a profoundly unexpected phenotype. The chronic symptoms of this disorder, termed PrP systemic amyloidosis, can be very disabling, and are comparable to familial amyloid polyneuropathy (FAP) caused by transthyretin mutations. Patients require symptomatic therapies directed towards control of nausea, diarrhoea, incontinence, neuropathic pain and postural hypotension. Although the potential transmissibility of this new prion disease is probably extremely low, we advocate PrP gene analysis before biopsy in the investigation of peripheral and autonomic neuropathies, or for patients with unexplained diarrhoea and neuropathy. Prion diseases and the FAPs both display prominent effects of mutation type on clinical presentation and patterns of pathology—a fascinating but unexplained observation. Several neurodegenerative diseases associated with central protein misfolding, such as Huntington and Parkinson diseases, also have under-recognized peripheral components. Most of the familial amyloidoses can be explained by known gene mutations, but amino acid variants in proteins involved in other central neurodegenerative diseases might direct the initial pathology to the periphery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Frangione, B. et al. Familial cerebral amyloid angiopathy related to stroke and dementia. Amyloid 8 (Suppl. 1), 36–42 (2001).
Collinge, J. Prion diseases of humans and animals: their causes and molecular basis. Annu. Rev. Neurosci. 24, 519–550 (2001).
Revesz, T. et al. Genetics and molecular pathogenesis of sporadic and hereditary cerebral amyloid angiopathies. Acta Neuropathol. 118, 115–130 (2009).
Plante-Bordeneuve, V. & Said, G. Familial amyloid polyneuropathy. Lancet Neurol. 10, 1086–1097 (2011).
Prusiner, S. B. Novel proteinaceous infectious particles cause scrapie. Science 216, 136–144 (1982).
Prusiner, S. B. et al. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell 35, 349–358 (1983).
Jucker, M. & Walker, L. C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501, 45–51 (2013).
Costa, P. P., Figueira, A. S. & Bravo, F. R. Amyloid fibril protein related to pre-albumin in familial amyloidotic polyneuropathy. Proc. Natl Acad. Sci. USA 75, 4499–4503 (1978).
Van Allen, M. W., Frohlich, H. A. & Davis, J. R. Inherited predisposition to generalized amyloidosis. Clinical and pathological study of a family with neuropathy nephropathy and peptic ulcer. Neurology 19, 10–25 (1969).
Levy, E. et al. Mutation in gelsolin gene in Finnish hereditary amyloidosis. J. Exp. Med. 172, 1865–1867 (1990).
Valleix, S. et al. Hereditary systemic amyloidosis due to Asp76Asn variant β2-microglobulin. N. Engl. J. Med. 366, 2276–2283 (2012).
Andrade, C. A peculiar form of peripheral neuropathy; familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain 75, 408–427 (1952).
Meretoja, J. Familial systemic paramyloidosis with lattice dystrophy of the cornea, progressive cranial neuropathy, skin changes and various internal symptoms. A previously unrecognized heritable syndrome. Ann. Clin. Res. 1, 314–324 (1969).
Mead, S. et al. A novel prion disease associated with diarrhea and autonomic neuropathy. N. Engl. J. Med. 369, 1904–1914 (2013).
Fraser, H. & Dickinson, A. G. Pathogenesis of scrapie in the mouse: the role of the spleen. Nature 226, 462–463 (1970).
Aguzzi, A., Nuvolone, M. & Zhu, C. The immunobiology of prion diseases. Nat. Rev. Immunol. 13, 888–902 (2013).
Miller, M. W. & Williams, E. S. Prion disease: horizontal prion transmission in mule deer. Nature 425, 35–36 (2003).
Seidel, B. et al. Scrapie agent (strain 263K) can transmit disease via the oral route after persistence in soil over years. PLoS ONE 2, e435 (2007).
Brown, P. et al. Iatrogenic Creutzfeldt–Jakob disease at the millennium. Neurology 55, 1075–1081 (2000).
Collinge, J. Variant Creutzfeldt–Jakob disease. Lancet 354, 317–323 (1999).
Whitfield, J. T., Pako, W. H., Collinge, J. & Alpers, M. P. Mortuary rites of the South Fore and kuru. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 3721–3724 (2008).
Collinge, J. et al. Kuru in the 21st century—an acquired human prion disease with very long incubation periods. Lancet 367, 2068–2074 (2006).
Glatzel, M., Abela, E., Maissen, M. & Aguzzi, A. Extraneural pathologic prion protein in sporadic Creutzfeldt–Jakob disease. N. Engl. J. Med. 349, 1812–1820 (2003).
Kitamoto, T., Muramoto, T., Mohri, S., Doh-ura, K. & Tateishi, J. Abnormal isoform of prion protein accumulates in follicular dendritic cells in mice with Creutzfeldt–Jakob disease. J. Virol. 65, 6292–6295 (1991).
Castro-Seoane, R. et al. Plasmacytoid dendritic cells sequester high prion titres at early stages of prion infection. PLoS Pathog. 8, e1002538 (2012).
Kimberlin, R. H. & Walker, C. A. The role of the spleen in the neuroinvasion of scrapie in mice. Virus Res. 12, 201–211 (1989).
Hill, A. F. et al. Investigation of variant Creutzfeldt–Jakob disease and other human prion diseases with tonsil biopsy samples. Lancet 353, 183–189 (1999).
Gill, O. N. et al. Prevalent abnormal prion protein in human appendixes after bovine spongiform encephalopathy epizootic: large scale survey. BMJ 347, f5675 (2013).
Peden, A. et al. Variant CJD infection in the spleen of a neurologically asymptomatic UK adult patient with haemophilia. Haemophilia 16, 296–304 (2010).
Peden, A. H., Head, M. W., Ritchie, D. L., Bell, J. E. & Ironside, J. W. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet 364, 527–529 (2004).
Llewelyn, C. A. et al. Possible transmission of variant Creutzfeldt–Jakob disease by blood transfusion. Lancet 363, 417–421 (2004).
Wroe, S. J. et al. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt–Jakob disease associated with blood transfusion: a case report. Lancet 368, 2061–2067 (2006).
Mead, S. Prion disease genetics. Eur. J. Hum. Genet. 14, 273–281 (2006).
Kitamoto, T., Iizuka, R. & Tateishi, J. An amber mutation of prion protein in Gerstmann–Straussler syndrome with mutant PrP plaques. Biochem. Biophys. Res. Commun. 192, 525–531 (1993).
Ghetti, B. et al. Vascular variant of prion protein cerebral amyloidosis with tau-positive neurofibrillary tangles: the phenotype of the stop codon 145 mutation in PRNP. Proc. Natl Acad. Sci. USA 93, 744–748 (1996).
Jayadev, S. et al. Familial prion disease with Alzheimer disease-like tau pathology and clinical phenotype. Ann. Neurol. 69, 712–720 (2011).
Jansen, C. et al. Prion protein amyloidosis with divergent phenotype associated with two novel nonsense mutations in PRNP. Acta Neuropathol. 119, 189–197 (2010).
Finckh, U. et al. High prevalence of pathogenic mutations in patients with early-onset dementia detected by sequence analyses of four different genes. Am. J. Hum. Genet. 66, 110–117 (2000).
Matsuzono, K. et al. A novel familial prion disease causing pan-autonomic-sensory neuropathy and cognitive impairment. Eur. J. Neurol. 20, e67–e69 (2013).
Themistocleous, A. C. et al. Late onset hereditary sensory and autonomic neuropathy with cognitive impairment associated with Y163X prion mutation. J. Neurol. 261, 2230–2233 (2014).
Capellari, S. et al. Prion disease associated with diarrhea and autonomic neuropathy: phenotypic and genetic characterization of an Italian family. Prion 8 (Suppl. 1), 127 (2014).
Montagna, P., Gambetti, P., Cortelli, P. & Lugaresi, E. Familial and sporadic fatal insomnia. Lancet Neurol. 2, 167–176 (2003).
Medori, R. et al. Fatal familial insomnia, a prion disease with a mutation at codon 178 of the prion protein gene. N. Engl. J. Med. 326, 444–449 (1992).
Cortelli, P. et al. Cardiovascular dysautonomia in fatal familial insomnia. Clin. Auton. Res. 1, 15–21 (1991).
Portaluppi, F. et al. Diurnal blood pressure variation and hormonal correlates in fatal familial insomnia. Hypertension 23, 569–576 (1994).
Auer-Grumbach, M. Hereditary sensory and autonomic neuropathies. Handb. Clin. Neurol. 115, 893–906 (2013).
Klein, C. J. et al. Mutations in DNMT1 cause hereditary sensory neuropathy with dementia and hearing loss. Nat. Genet. 43, 595–600 (2011).
Adams, D., Lozeron, P. & Lacroix, C. Amyloid neuropathies. Curr. Opin. Neurol. 25, 564–572 (2012).
Cappellari, M. et al. Variable presentations of TTR-related familial amyloid polyneuropathy in seventeen patients. J. Peripher. Nerv. Sys. 16, 119–129 (2011).
Vidal, R. et al. A stop-codon mutation in the BRI gene associated with familial British dementia. Nature 399, 776–781 (1999).
Levy, E. et al. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science 248, 1124–1126 (1990).
Beck, J. et al. Validation of next-generation sequencing technologies in genetic diagnosis of dementia. Neurobiol. Aging 35, 261–265 (2014).
Guerreiro, R., Bras, J., Hardy, J. & Singleton, A. Next generation sequencing techniques in neurological diseases: redefining clinical and molecular associations. Hum. Mol. Genet. 23, R47–R53 (2014).
Gibbs, C. J. Jr et al. Transmission of Creutzfeldt–Jakob disease to a chimpanzee by electrodes contaminated during neurosurgery. J. Neurol. Neurosurg. Psychiatry 57, 757–758 (1994).
van der Burg, J. M. et al. Gastrointestinal dysfunction contributes to weight loss in Huntington's disease mice. Neurobiol. Dis. 44, 1–8 (2011).
van der Burg, J. M., Bjorkqvist, M. & Brundin, P. Beyond the brain: widespread pathology in Huntington's disease. Lancet Neurol. 8, 765–774 (2009).
Sassone, J., Colciago, C., Cislaghi, G., Silani, V. & Ciammola, A. Huntington's disease: the current state of research with peripheral tissues. Exp. Neurol. 219, 385–397 (2009).
Hilton, D. et al. Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson's disease. Acta Neuropathol. 127, 235–241 (2014).
Olanow, C. W., Wakeman, D. R. & Kordower, J. H. Peripheral alpha-synuclein and Parkinson's disease. Mov. Disord. 29, 963–966 (2014).
Beach, T. G. et al. Submandibular gland biopsy for the diagnosis of Parkinson disease. J. Neuropathol. Exp. Neurol. 72, 130–136 (2013).
Collinge, J. Prion strain mutation and selection. Science 328, 1111–1112 (2010).
Collinge, J. & Clarke, A. R. A general model of prion strains and their pathogenicity. Science 318, 930–936 (2007).
Tanaka, M., Chien, P., Yonekura, K. & Weissman, J. S. Mechanism of cross-species prion transmission an infectious conformation compatible withtwo highly divergent yeast prion proteins. Cell 121, 49–62 (2005).
Jacobson, D. R. et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N. Engl. J. Med. 336, 466–473 (1997).
Raimondi, S. et al. Effects of the known pathogenic mutations on the aggregation pathway of the amyloidogenic peptide of apolipoprotein A-I. J. Mol. Biol. 407, 465–476 (2011).
Wadsworth, J. D. et al. Tissue distribution of protease resistant prion protein in variant Creutzfeldt–Jakob disease using a highly sensitive immunoblotting assay. Lancet 358, 171–180 (2001).
Minikel, E. et al. Prion protein genetic variation in 50,000 humans. Prion 8 (Suppl. 1), 115 (2014).
Exome Aggregation Consortium. ExAC Browser (Beta) [online], (2014).
Acknowledgements
S.M. is funded by core support for the UK Medical Research Council (MRC) Prion Unit. M.M.R. is grateful to the MRC for an MRC Centre Grant (G0601943), and the US National Institutes of Neurological Diseases and Stroke and the Office of Rare Diseases (U54NS065712) for their support. This work was undertaken at University College London Hospitals and University College London, which received a proportion of funding from the UK Department of Health's National Institute for Health Research Biomedical Research Centres funding scheme.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to researching data for the article, discussion of content, writing the article, and review and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Mead, S., Reilly, M. A new prion disease: relationship with central and peripheral amyloidoses. Nat Rev Neurol 11, 90–97 (2015). https://doi.org/10.1038/nrneurol.2014.263
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2014.263
This article is cited by
-
Prion strains viewed through the lens of cryo-EM
Cell and Tissue Research (2023)
-
Bank vole prion protein extends the use of RT-QuIC assays to detect prions in a range of inherited prion diseases
Scientific Reports (2021)
-
Huntington’s disease: lessons from prion disorders
Journal of Neurology (2021)
-
Structural features distinguishing infectious ex vivo mammalian prions from non-infectious fibrillar assemblies generated in vitro
Scientific Reports (2019)
-
Cerebral Amyloid Angiopathy, Alzheimer’s Disease and MicroRNA: miRNA as Diagnostic Biomarkers and Potential Therapeutic Targets
NeuroMolecular Medicine (2019)