Key Points
-
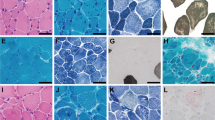
Congenital myopathies are clinically and genetically heterogeneous conditions characterized by muscle weakness and distinctive structural abnormalities in muscle biopsy samples
-
Clinically, congenital myopathies have a stable or slowly progressive course, and the severity varies depending on the causative mutation
-
More than 20 genes have been implicated in congenital myopathies
-
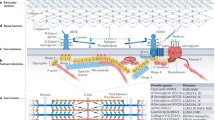
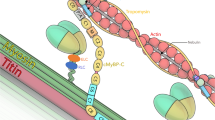
The most commonly affected genes encode proteins involved in skeletal muscle Ca2+ homeostasis, excitation–contraction coupling and thin–thick filament assembly and interactions
-
Management of congenital myopathies is largely supportive, although experimental therapeutic approaches are reaching the clinical trial stage
Abstract
The congenital myopathies are a group of early-onset, non-dystrophic neuromuscular conditions with characteristic muscle biopsy findings, variable severity and a stable or slowly progressive course. Pronounced weakness in axial and proximal muscle groups is a common feature, and involvement of extraocular, cardiorespiratory and/or distal muscles can implicate specific genetic defects. Central core disease (CCD), multi-minicore disease (MmD), centronuclear myopathy (CNM) and nemaline myopathy were among the first congenital myopathies to be reported, and they still represent the main diagnostic categories. However, these entities seem to belong to a much wider phenotypic spectrum. To date, congenital myopathies have been attributed to mutations in over 20 genes, which encode proteins implicated in skeletal muscle Ca2+ homeostasis, excitation–contraction coupling, thin–thick filament assembly and interactions, and other mechanisms. RYR1 mutations are the most frequent genetic cause, and CCD and MmD are the most common subgroups. Next-generation sequencing has vastly improved mutation detection and has enabled the identification of novel genetic backgrounds. At present, management of congenital myopathies is largely supportive, although new therapeutic approaches are reaching the clinical trial stage.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Magee, K. R. & Shy, G. M. A new congenital non-progressive myopathy. Brain 79, 610–621 (1956).
Engel, A. G., Gomez, M. R. & Groover, R. V. Multicore disease. A recently recognized congenital myopathy associated with multifocal degeneration of muscle fibers. Mayo Clin. Proc. 46, 666–681 (1971).
Spiro, A. J., Shy, G. M. & Gonatas, N. K. Myotubular myopathy. Persistence of fetal muscle in an adolescent boy. Arch. Neurol. 14, 1–14 (1966).
Shy, G. M., Engel, W. K., Somers, J. E. & Wanko, T. Nemaline myopathy. a new congenital myopathy. Brain 86, 793–810 (1963).
Lopez, R. J. et al. An RYR1 mutation associated with malignant hyperthermia is also associated with bleeding abnormalities. Sci. Signal. 9, ra68 (2016).
Jungbluth, H. Myopathology in times of modern imaging. Neuropathol. Appl. Neurobiol. 43, 24–43 (2017).
Snoeck, M. et al. RYR1-related myopathies: a wide spectrum of phenotypes throughout life. Eur. J. Neurol. 22, 1094–1112 (2015).
Jungbluth, H. & Voermans, N. C. Congenital myopathies: not only a paediatric topic. Curr. Opin. Neurol. 29, 642–650 (2016).
Quane, K. A. et al. Mutations in the ryanodine receptor gene in central core disease and malignant hyperthermia. Nat. Genet. 5, 51–55 (1993).
Fujii, J. et al. Identification of a mutation in porcine ryanodine receptor associated with malignant hyperthermia. Science 253, 448–451 (1991).
Biancalana, V. & Laporte, J. Diagnostic use of massively parallel sequencing in neuromuscular diseases: towards an integrated diagnosis. J. Neuromuscul. Dis. 2, 193–203 (2015).
Jungbluth, H., Sewry, C. A. & Muntoni, F. Core myopathies. Semin. Pediatr. Neurol. 18, 239–249 (2011).
Dubowitz, V., Sewry, C. A. & Oldfors, A. Muscle Biopsy: a Practical Approach 4th edn (Saunders, 2013).
Amburgey, K. et al. Prevalence of congenital myopathies in a representative pediatric United States population. Ann. Neurol. 70, 662–665 (2011).
Hackman, P., Udd, B., Bonnemann, C. G., Ferreiro, A. & Titinopathy Database Consortium. 219th ENMC International Workshop Titinopathies International database of titin mutations and phenotypes, Heemskerk, The Netherlands, 29 April-1 May 2016. Neuromuscul. Disord. 27, 396–407 (2017).
Jungbluth, H. et al. Autosomal recessive inheritance of RYR1 mutations in a congenital myopathy with cores. Neurology 59, 284–287 (2002).
Jungbluth, H. et al. Minicore myopathy with ophthalmoplegia caused by mutations in the ryanodine receptor type 1 gene. Neurology 65, 1930–1935 (2005).
Klein, A. et al. Clinical and genetic findings in a large cohort of patients with ryanodine receptor 1 gene-associated myopathies. Hum. Mutat. 33, 981–988 (2012).
Ferreiro, A. et al. Mutations of the selenoprotein N gene, which is implicated in rigid spine muscular dystrophy, cause the classical phenotype of multiminicore disease: reassessing the nosology of early-onset myopathies. Am. J. Hum. Genet. 71, 739–749 (2002).
Cullup, T. et al. Mutations in MYH7 cause multi-minicore disease (MmD) with variable cardiac involvement. Neuromuscul. Disord. 22, 1096–1104 (2012).
Takayama, K. et al. Japanese multiple epidermal growth factor 10 (MEGF10) myopathy with novel mutations: a phenotype–genotype correlation. Neuromuscul. Disord. 26, 604–609 (2016).
Liewluck, T. et al. Adult-onset respiratory insufficiency, scoliosis, and distal joint hyperlaxity in patients with multiminicore disease due to novel Megf10 mutations. Muscle Nerve 53, 984–988 (2016).
Logan, C. V. et al. Mutations in MEGF10, a regulator of satellite cell myogenesis, cause early onset myopathy, areflexia, respiratory distress and dysphagia (EMARDD). Nat. Genet. 43, 1189–1192 (2011).
Boyden, S. E. et al. Mutations in the satellite cell gene MEGF10 cause a recessive congenital myopathy with minicores. Neurogenetics 13, 115–124 (2012).
Chauveau, C. et al. Recessive TTN truncating mutations define novel forms of core myopathy with heart disease. Hum. Mol. Genet. 23, 980–991 (2014).
Romero, N. B. et al. Dominant and recessive central core disease associated with RYR1 mutations and fetal akinesia. Brain 126, 2341–2349 (2003).
Scoto, M. et al. SEPN1-related myopathies: clinical course in a large cohort of patients. Neurology 76, 2073–2078 (2011).
Klein, A. et al. Muscle MRI in congenital myopathies due to ryanodine receptor type 1 gene mutations. Arch. Neurol. 68, 1171–1179 (2011).
Jungbluth, H. et al. Magnetic resonance imaging of muscle in congenital myopathies associated with RYR1 mutations. Neuromuscul. Disord. 14, 785–790 (2004).
Rosenberg, H., Davis, M., James, D., Pollock, N. & Stowell, K. Malignant hyperthermia. Orphanet J. Rare Dis. 2, 21 (2007).
Zhou, H. et al. Characterization of recessive RYR1 mutations in core myopathies. Hum. Mol. Genet. 15, 2791–2803 (2006).
Kraeva, N. et al. Compound RYR1 heterozygosity resulting in a complex phenotype of malignant hyperthermia susceptibility and a core myopathy. Neuromuscul. Disord. 25, 567–576 (2015).
Dowling, J. J. et al. King–Denborough syndrome with and without mutations in the skeletal muscle ryanodine receptor (RYR1) gene. Neuromuscul. Disord. 21, 420–427 (2011).
Horstick, E. J. et al. Stac3 is a component of the excitation–contraction coupling machinery and mutated in Native American myopathy. Nat. Commun. 4, 1952 (2013).
Dlamini, N. et al. Mutations in RYR1 are a common cause of exertional myalgia and rhabdomyolysis. Neuromuscul. Disord. 23, 540–548 (2013).
Bethlem, J., van Gool, J., Hulsmann, W. C. & Meijer, A. E. Familial non-progressive myopathy with muscle cramps after exercise. A new disease associated with cores in the muscle fibres. Brain 89, 569–588 (1966).
Løseth, S. et al. A novel late-onset axial myopathy associated with mutations in the skeletal muscle ryanodine receptor (RYR1) gene. J. Neurol. 260, 1504–1510 (2013).
Jungbluth, H. et al. Late-onset axial myopathy with cores due to a novel heterozygous dominant mutation in the skeletal muscle ryanodine receptor (RYR1) gene. Neuromuscul. Disord. 19, 344–347 (2009).
Jungbluth, H., Wallgren-Pettersson, C. & Laporte, J. Centronuclear (myotubular) myopathy. Orphanet J. Rare Dis. 3, 26 (2008).
Laporte, J. et al. A gene mutated in X-linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast. Nat. Genet. 13, 175–182 (1996).
Bitoun, M. et al. Mutations in dynamin 2 cause dominant centronuclear myopathy. Nat. Genet. 37, 1207–1209 (2005).
Böhm, J. et al. Adult-onset autosomal dominant centronuclear myopathy due to BIN1 mutations. Brain 137, 3160–3170 (2014).
Wilmshurst, J. M. et al. RYR1 mutations are a common cause of congenital myopathies with central nuclei. Ann. Neurol. 68, 717–726 (2010).
Nicot, A. S. et al. Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nat. Genet. 39, 1134–1139 (2007).
Ceyhan-Birsoy, O. et al. Recessive truncating titin gene. TTN, mutations presenting as centronuclear myopathy. Neurology 81, 1205–1214 (2013).
Agrawal, P. B. et al. SPEG interacts with myotubularin, and its deficiency causes centronuclear myopathy with dilated cardiomyopathy. Am. J. Hum. Genet. 95, 218–226 (2014).
Majczenko, K. et al. Dominant mutation of CCDC78 in a unique congenital myopathy with prominent internal nuclei and atypical cores. Am. J. Hum. Genet. 91, 365–371 (2012).
Tosch, V. et al. A novel PtdIns3P and PtdIns(3,5)P2 phosphatase with an inactivating variant in centronuclear myopathy. Hum. Mol. Genet. 15, 3098–3106 (2006).
Bevilacqua, J. A. et al. “Necklace” fibers, a new histological marker of late-onset MTM1-related centronuclear myopathy. Acta Neuropathol. 117, 283–291 (2009).
Liewluck, T., Lovell, T. L., Bite, A. V. & Engel, A. G. Sporadic centronuclear myopathy with muscle pseudohypertrophy, neutropenia, and necklace fibers due to a DNM2 mutation. Neuromuscul. Disord. 20, 801–804 (2010).
Toussaint, A. et al. Defects in amphiphysin 2 (BIN1) and triads in several forms of centronuclear myopathies. Acta Neuropathol. 121, 253–266 (2011).
Romero, N. B. Centronuclear myopathies: a widening concept. Neuromuscul. Disord. 20, 223–228 (2010).
Herman, G. E., Finegold, M., Zhao, W., de Gouyon, B. & Metzenberg, A. Medical complications in long-term survivors with X-linked myotubular myopathy. J. Pediatr. 134, 206–214 (1999).
Bohm, J. et al. Mutation spectrum in the large GTPase dynamin 2, and genotype–phenotype correlation in autosomal dominant centronuclear myopathy. Hum. Mutat. 33, 949–959 (2012).
Bitoun, M. et al. Dynamin 2 mutations cause sporadic centronuclear myopathy with neonatal onset. Ann. Neurol. 62, 666–670 (2007).
Jungbluth, H. et al. Centronuclear myopathy with cataracts due to a novel dynamin 2 (DNM2) mutation. Neuromuscul. Disord. 20, 49–52 (2010).
Zuchner, S. et al. Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot–Marie–Tooth disease. Nat. Genet. 37, 289–294 (2005).
Jungbluth, H., Wallgren-Pettersson, C. & Laporte, J. F. 198th ENMC International Workshop: 7th Workshop on Centronuclear (Myotubular) Myopathies, 31st May–2nd June 2013, Naarden, The Netherlands. Neuromuscul. Disord. 23, 1033–1043 (2013).
Pelin, K. et al. Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc. Natl Acad. Sci. USA 96, 2305–2310 (1999).
Pelin, K. et al. Nebulin mutations in autosomal recessive nemaline myopathy: an update. Neuromuscul. Disord. 12, 680–686 (2002).
Nowak, K. J. et al. Mutations in the skeletal muscle α-actin gene in patients with actin myopathy and nemaline myopathy. Nat. Genet. 23, 208–212 (1999).
Laing, N. G. et al. A mutation in the α tropomyosin gene TPM3 associated with autosomal dominant nemaline myopathy. Nat. Genet. 9, 75–79 (1995).
Donner, K. et al. Mutations in the β-tropomyosin (TPM2) gene — a rare cause of nemaline myopathy. Neuromuscul. Disord. 12, 151–158 (2002).
Sambuughin, N. et al. Dominant mutations in KBTBD13, a member of the BTB/Kelch family, cause nemaline myopathy with cores. Am. J. Hum. Genet. 87, 842–847 (2010).
Laing, N. G. et al. Mutations and polymorphisms of the skeletal muscle α-actin gene (ACTA1). Hum. Mutat. 30, 1267–1277 (2009).
Lehtokari, V. L. et al. Identification of a founder mutation in TPM3 in nemaline myopathy patients of Turkish origin. Eur. J. Hum. Genet. 16, 1055–1061 (2008).
Monnier, N. et al. Absence of β-tropomyosin is a new cause of Escobar syndrome associated with nemaline myopathy. Neuromuscul. Disord. 19, 118–123 (2009).
Johnston, J. J. et al. A novel nemaline myopathy in the Amish caused by a mutation in troponin T1. Am. J. Hum. Genet. 67, 814–821 (2000).
Agrawal, P. B. et al. Nemaline myopathy with minicores caused by mutation of the CFL2 gene encoding the skeletal muscle actin-binding protein, cofilin-2. Am. J. Hum. Genet. 80, 162–167 (2007).
Ravenscroft, G. et al. Mutations in KLHL40 are a frequent cause of severe autosomal-recessive nemaline myopathy. Am. J. Hum. Genet. 93, 6–18 (2013).
Gupta, V. A. et al. Identification of KLHL41 mutations implicates BTB-Kelch-mediated ubiquitination as an alternate pathway to myofibrillar disruption in nemaline myopathy. Am. J. Hum. Genet. 93, 1108–1117 (2013).
Yuen, M. et al. Leiomodin-3 dysfunction results in thin filament disorganization and nemaline myopathy. J. Clin. Invest. 124, 4693–4708 (2014).
Lornage, X. et al. Recessive MYPN mutations cause cap myopathy with occasional nemaline rods. Ann. Neurol. 81, 467–473 (2017).
Miyatake, S. et al. Biallelic mutations in MYPN, encoding myopalladin, are associated with childhood-onset, slowly progressive nemaline myopathy. Am. J. Hum. Genet. 100, 169–178 (2017).
Malfatti, E. et al. A premature stop codon in MYO18B is associated with severe nemaline myopathy with cardiomyopathy. J. Neuromuscul. Dis. 2, 219–227 (2015).
Domazetovska, A. et al. Intranuclear rod myopathy: molecular pathogenesis and mechanisms of weakness. Ann. Neurol. 62, 597–608 (2007).
Ryan, M. M. et al. Nemaline myopathy: a clinical study of 143 cases. Ann. Neurol. 50, 312–320 (2001).
Feng, J. J. & Marston, S. Genotype–phenotype correlations in ACTA1 mutations that cause congenital myopathies. Neuromuscul. Disord. 19, 6–16 (2009).
Witting, N., Werlauff, U., Duno, M. & Vissing, J. Prevalence and phenotypes of congenital myopathy due to α-actin 1 gene mutations. Muscle Nerve 53, 388–393 (2016).
Jungbluth, H. et al. Mild phenotype of nemaline myopathy with sleep hypoventilation due to a mutation in the skeletal muscle α-actin (ACTA1) gene. Neuromuscul. Disord. 11, 35–40 (2001).
Sambuughin, N. et al. KBTBD13 interacts with Cullin 3 to form a functional ubiquitin ligase. Biochem. Biophys. Res. Commun. 421, 743–749 (2012).
Davidson, A. E. et al. Novel deletion of lysine 7 expands the clinical, histopathological and genetic spectrum of TPM2-related myopathies. Brain 136, 508–521 (2013).
Jungbluth, H. et al. Magnetic resonance imaging of muscle in nemaline myopathy. Neuromuscul. Disord. 14, 779–784 (2004).
Sato, I. et al. Congenital neuromuscular disease with uniform type 1 fiber and RYR1 mutation. Neurology 70, 114–122 (2008).
Muhammad, E. et al. Congenital myopathy is caused by mutation of HACD1. Hum. Mol. Genet. 22, 5229–5236 (2013).
Maurer, M. et al. Centronuclear myopathy in Labrador retrievers: a recent founder mutation in the PTPLA gene has rapidly disseminated worldwide. PLoS ONE 7, e46408 (2012).
Walmsley, G. L. et al. Progressive structural defects in canine centronuclear myopathy indicate a role for HACD1 in maintaining skeletal muscle membrane systems. Am. J. Pathol. 187, 441–456 (2017).
Clarke, N. F. et al. Mutations in TPM3 are a common cause of congenital fiber type disproportion. Ann. Neurol. 63, 329–337 (2008).
Munot, P. et al. Congenital fibre type disproportion associated with mutations in the tropomyosin 3 (TPM3) gene mimicking congenital myasthenia. Neuromuscul. Disord. 20, 796–800 (2010).
Clarke, N. F. et al. Recessive mutations in RYR1 are a common cause of congenital fiber type disproportion. Hum. Mutat. 31, E1544–E1550 (2010).
Laing, N. G. et al. Actin mutations are one cause of congenital fibre type disproportion. Ann. Neurol. 56, 689–694 (2004).
Clarke, N. F. et al. SEPN1: associated with congenital fiber-type disproportion and insulin resistance. Ann. Neurol. 59, 546–552 (2006).
Lamont, P. J. et al. Novel mutations widen the phenotypic spectrum of slow skeletal/β-cardiac myosin (MYH7) distal myopathy. Hum. Mutat. 35, 868–879 (2014).
Vallat, J. M. et al. Coexistence of minicores, cores, and rods in the same muscle biopsy. A new example of mixed congenital myopathy. Acta Neuropathol. 58, 229–232 (1982).
Schartner, V. et al. Dihydropyridine receptor (DHPR. CACNA1S) congenital myopathy. Acta Neuropathol. 133, 517–533 (2017).
Monnier, N. et al. Presence of two different genetic traits in malignant hyperthermia families: implication for genetic analysis, diagnosis, and incidence of malignant hyperthermia susceptibility. Anesthesiology 97, 1067–1074 (2002).
Jurkat-Rott, K. et al. A calcium channel mutation causing hypokalemic periodic paralysis. Hum. Mol. Genet. 3, 1415–1419 (1994).
O'Grady, G. L. et al. Variants in the oxidoreductase PYROXD1 cause early-onset myopathy with internalized nuclei and myofibrillar disorganization. Am. J. Hum. Genet. 99, 1086–1105 (2016).
Tajsharghi, H. & Oldfors, A. Myosinopathies: pathology and mechanisms. Acta Neuropathol. 125, 3–18 (2013).
Tajsharghi, H. et al. Human disease caused by loss of fast IIa myosin heavy chain due to recessive MYH2 mutations. Brain 133, 1451–1459 (2010).
Martinsson, T. et al. Autosomal dominant myopathy: missense mutation (Glu-706 → Lys) in the myosin heavy chain IIa gene. Proc. Natl Acad. Sci. USA 97, 14614–14619 (2000).
Willis, T. et al. A novel MYH2 mutation in family members presenting with congenital myopathy, ophthalmoplegia and facial weakness. J. Neurol. 263, 1427–1433 (2016).
Tsabari, R., Daum, H., Kerem, E., Fellig, Y. & Dor, T. Congenital myopathy due to myosin heavy chain 2 mutation presenting as chronic aspiration pneumonia in infancy. Neuromuscul. Disord. 27, 947–950 (2017).
McMillin, M. J. et al. Mutations in ECEL1 cause distal arthrogryposis type 5D. Am. J. Hum. Genet. 92, 150–156 (2013).
Dieterich, K. et al. The neuronal endopeptidase ECEL1 is associated with a distinct form of recessive distal arthrogryposis. Hum. Mol. Genet. 22, 1483–1492 (2013).
Shaaban, S. et al. Expanding the phenotypic spectrum of ECEL1-related congenital contracture syndromes. Clin. Genet. 85, 562–567 (2014).
Todd, E. J. et al. Next generation sequencing in a large cohort of patients presenting with neuromuscular disease before or at birth. Orphanet J. Rare Dis. 10, 148 (2015).
Bayram, Y. et al. Molecular etiology of arthrogryposis in multiple families of mostly Turkish origin. J. Clin. Invest. 126, 762–778 (2016).
Coste, B. et al. Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of distal arthrogryposis. Proc. Natl Acad. Sci. USA 110, 4667–4672 (2013).
Zaharieva, I. T. et al. Loss-of-function mutations in SCN4A cause severe foetal hypokinesia or 'classical' congenital myopathy. Brain 139, 674–691 (2016).
Singh, R. R. et al. Mutations in SCN4A: a rare but treatable cause of recurrent life-threatening laryngospasm. Pediatrics 134, e1447–e1450 (2014).
Bharucha-Goebel, D. X. et al. Severe congenital RYR1-associated myopathy: the expanding clinicopathologic and genetic spectrum. Neurology 80, 1584–1589 (2013).
Schessl, J. et al. MRI in DNM2-related centronuclear myopathy: evidence for highly selective muscle involvement. Neuromuscul. Disord. 17, 28–32 (2007).
Clarke, N. F. et al. Cap disease due to mutation of the beta-tropomyosin gene (TPM2). Neuromuscul. Disord. 19, 348–351 (2009).
Lehtokari, V. L. et al. Cap disease caused by heterozygous deletion of the β-tropomyosin gene TPM2. Neuromuscul. Disord. 17, 433–442 (2007).
Sewry, C. A., Holton, J. L., Dick, D. J., Muntoni, F. & Hanna, M. G. Zebra body myopathy is caused by a mutation in the skeletal muscle actin gene (ACTA1). Neuromuscul. Disord. 25, 388–391 (2015).
Lacruz, R. S. & Feske, S. Diseases caused by mutations in ORAI1 and STIM1. Ann. NY Acad. Sci. 1356, 45–79 (2015).
Gordon, C. P. & Litz, S. Multicore myopathy in a patient with anhidrotic ectodermal dysplasia. Can. J. Anaesth. 39, 966–968 (1992).
Engel, A. G., Redhage, K. R., Tester, D. J., Ackerman, M. J. & Selcen, D. Congenital myopathy associated with the triadin knockout syndrome. Neurology 88, 1153–1156 (2017).
Altmann, H. M. et al. Homozygous/compound heterozygous triadin mutations associated with autosomal-recessive long-QT syndrome and pediatric sudden cardiac arrest: elucidation of the triadin knockout syndrome. Circulation 131, 2051–2060 (2015).
Olive, M. et al. New cardiac and skeletal protein aggregate myopathy associated with combined MuRF1 and MuRF3 mutations. Hum. Mol. Genet. 24, 6264 (2015).
Zhang, L., Kelley, J., Schmeisser, G., Kobayashi, Y. M. & Jones, L. R. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J. Biol. Chem. 272, 23389–23397 (1997).
Park, H. et al. Comparing skeletal and cardiac calsequestrin structures and their calcium binding: a proposed mechanism for coupled calcium binding and protein polymerization. J. Biol. Chem. 279, 18026–18033 (2004).
Costello, B. et al. Characterization of the junctional face membrane from terminal cisternae of sarcoplasmic reticulum. J. Cell Biol. 103, 741–753 (1986).
Treves, S. et al. Minor sarcoplasmic reticulum membrane components that modulate excitation–contraction coupling in striated muscles. J. Physiol. 587, 3071–3079 (2009).
Rios, E. & Gyorke, S. Calsequestrin, triadin and more: the molecules that modulate calcium release in cardiac and skeletal muscle. J. Physiol. 587, 3069–3070 (2009).
Guo, W. & Campbell, K. P. Association of triadin with the ryanodine receptor and calsequestrin in the lumen of the sarcoplasmic reticulum. J. Biol. Chem. 270, 9027–9030 (1995).
Wium, E., Dulhunty, A. F. & Beard, N. A. Three residues in the luminal domain of triadin impact on Trisk 95 activation of skeletal muscle ryanodine receptors. Pflugers Arch. 468, 1985–1994 (2016).
Caswell, A. H., Motoike, H. K., Fan, H. & Brandt, N. R. Location of ryanodine receptor binding site on skeletal muscle triadin. Biochemistry 38, 90–97 (1999).
Groh, S. et al. Functional interaction of the cytoplasmic domain of triadin with the skeletal ryanodine receptor. J. Biol. Chem. 274, 12278–12283 (1999).
Goonasekera, S. A. et al. Triadin binding to the C-terminal luminal loop of the ryanodine receptor is important for skeletal muscle excitation contraction coupling. J. Gen. Physiol. 130, 365–378 (2007).
Gordon, A. M., Homsher, E. & Regnier, M. Regulation of contraction in striated muscle. Physiol. Rev. 80, 853–924 (2000).
Abu-Abed, M., Mal, T. K., Kainosho, M., MacLennan, D. H. & Ikura, M. Characterization of the ATP-binding domain of the sarco(endo)plasmic reticulum Ca2+-ATPase: probing nucleotide binding by multidimensional NMR. Biochemistry 41, 1156–1164 (2002).
MacLennan, D. H., Asahi, M. & Tupling, A. R. The regulation of SERCA-type pumps by phospholamban and sarcolipin. Ann. NY Acad. Sci. 986, 472–480 (2003).
MacLennan, D. H. & Kranias, E. G. Phospholamban: a crucial regulator of cardiac contractility. Nat. Rev. Mol. Cell. Biol. 4, 566–577 (2003).
Asahi, M. et al. Sarcolipin regulates sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) by binding to transmembrane helices alone or in association with phospholamban. Proc. Natl Acad. Sci. USA 100, 5040–5045 (2003).
Kurebayashi, N. & Ogawa, Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J. Physiol. 533, 185–199 (2001).
Cherednichenko, G. et al. Conformational activation of Ca2+ entry by depolarization of skeletal myotubes. Proc. Natl Acad. Sci. USA 101, 15793–15798 (2004).
Launikonis, B. S. & Rios, E. Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J. Physiol. 583, 81–97 (2007).
Stiber, J. et al. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat. Cell Biol. 10, 688–697 (2008).
Peinelt, C. et al. Amplification of CRAC current by STIM1 and CRACM1 (Orai1). Nat. Cell Biol. 8, 771–773 (2006).
Treves, S., Jungbluth, H., Muntoni, F. & Zorzato, F. Congenital muscle disorders with cores: the ryanodine receptor calcium channel paradigm. Curr. Opin. Pharmacol. 8, 319–326 (2008).
Hwang, J. H., Zorzato, F., Clarke, N. F. & Treves, S. Mapping domains and mutations on the skeletal muscle ryanodine receptor channel. Trends Mol. Med. 18, 644–657 (2012).
Maclennan, D. H. & Zvaritch, E. Mechanistic models for muscle diseases and disorders originating in the sarcoplasmic reticulum. Biochim. Biophys. Acta 1813, 948–964 (2011).
Hirata, H. et al. Zebrafish relatively relaxed mutants have a ryanodine receptor defect, show slow swimming and provide a model of multi-minicore disease. Development 134, 2771–2781 (2007).
Zhou, H. et al. RyR1 deficiency in congenital myopathies disrupts excitation–contraction coupling. Hum. Mutat. 34, 986–996 (2013).
Zhou, H. et al. Epigenetic allele silencing unveils recessive RYR1 mutations in core myopathies. Am. J. Hum. Genet. 79, 859–868 (2006).
Ducreux, S. et al. Functional properties of ryanodine receptors carrying three amino acid substitutions identified in patients affected by multi-minicore disease and central core disease, expressed in immortalized lymphocytes. Biochem. J. 395, 259–266 (2006).
Nelson, B. R. et al. Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility. Proc. Natl Acad. Sci. USA 110, 11881–11886 (2013).
Polster, A., Nelson, B. R., Olson, E. N. & Beam, K. G. Stac3 has a direct role in skeletal muscle-type excitation–contraction coupling that is disrupted by a myopathy-causing mutation. Proc. Natl Acad. Sci. USA 113, 10986–10991 (2016).
Bohm, J. et al. Constitutive activation of the calcium sensor STIM1 causes tubular-aggregate myopathy. Am. J. Hum. Genet. 92, 271–278 (2013).
Volkers, M. et al. Orai1 deficiency leads to heart failure and skeletal myopathy in zebrafish. J. Cell Sci. 125, 287–294 (2012).
Jurynec, M. J. et al. Selenoprotein N is required for ryanodine receptor calcium release channel activity in human and zebrafish muscle. Proc. Natl Acad. Sci. USA 105, 12485–12490 (2008).
Arbogast, S. et al. Oxidative stress in SEPN1-related myopathy: from pathophysiology to treatment. Ann. Neurol. 65, 677–686 (2009).
Jungbluth, H. & Gautel, M. Pathogenic mechanisms in centronuclear myopathies. Front. Aging Neurosci. 6, 339 (2014).
Cowling, B. S., Toussaint, A., Muller, J. & Laporte, J. Defective membrane remodeling in neuromuscular diseases: insights from animal models. PLoS Genet. 8, e1002595 (2012).
Bachmann, C. et al. Cellular, biochemical and molecular changes in muscles from patients with X-linked myotubular myopathy due to MTM1 mutations. Hum. Mol. Genet. 26, 320–332 (2017).
Wallgren-Pettersson, C., Sewry, C. A., Nowak, K. J. & Laing, N. G. Nemaline myopathies. Sem. Pediatr. Neurol. 18, 230–238 (2011).
Ravenscroft, G. et al. Mouse models of dominant ACTA1 disease recapitulate human disease and provide insight into therapies. Brain 134, 1101–1115 (2011).
Ravenscroft, G. et al. Actin nemaline myopathy mouse reproduces disease, suggests other actin disease phenotypes and provides cautionary note on muscle transgene expression. PLoS ONE 6, e28699 (2011).
Jain, R. K. et al. Nemaline myopathy with stiffness and hypertonia associated with an ACTA1 mutation. Neurology 78, 1100–1103 (2012).
Donkervoort, S. et al. TPM3 deletions cause a hypercontractile congenital muscle stiffness phenotype. Ann. Neurol. 78, 982–994 (2015).
Ochala, J. et al. Disrupted myosin cross-bridge cycling kinetics triggers muscle weakness in nebulin-related myopathy. FASEB J. 25, 1903–1913 (2011).
Marttila, M. et al. Nebulin interactions with actin and tropomyosin are altered by disease-causing mutations. Skelet. Muscle 4, 15 (2014).
de Winter, J. M. et al. Mutation-specific effects on thin filament length in thin filament myopathy. Ann. Neurol. 9, 959–969 (2016).
Ajima, R. et al. Deficiency of Myo18B in mice results in embryonic lethality with cardiac myofibrillar aberrations. Genes Cells 13, 987–999 (2008).
Gurung, R. et al. A zebrafish model for a human myopathy associated with mutation of the unconventional myosin MYO18B. Genetics 205, 725–735 (2017).
Gupta, V. A. & Beggs, A. H. Kelch proteins: emerging roles in skeletal muscle development and diseases. Skelet. Muscle 4, 11 (2014).
Garg, A. et al. KLHL40 deficiency destabilizes thin filament proteins and promotes nemaline myopathy. J. Clin. Invest. 124, 3529–3539 (2014).
Castets, P. et al. Satellite cell loss and impaired muscle regeneration in selenoprotein N deficiency. Hum. Mol. Genet. 20, 694–704 (2011).
Castets, P. et al. Selenoprotein N is dynamically expressed during mouse development and detected early in muscle precursors. BMC Dev. Biol. 9, 46 (2009).
Beggs, A. H. et al. MTM1 mutation associated with X-linked myotubular myopathy in Labrador Retrievers. Proc. Natl Acad. Sci. USA 107, 14697–14702 (2010).
Dowling, J. J., Low, S. E., Busta, A. S. & Feldman, E. L. Zebrafish MTMR14 is required for excitation–contraction coupling, developmental motor function and the regulation of autophagy. Hum. Mol. Genet. 19, 2668–2681 (2010).
Fetalvero, K. M. et al. Defective autophagy and mTORC1 signaling in myotubularin null mice. Mol. Cell. Biol. 33, 98–110 (2013).
Al-Qusairi, L. et al. Lack of myotubularin (MTM1) leads to muscle hypotrophy through unbalanced regulation of the autophagy and ubiquitin–proteasome pathways. FASEB J. 27, 3384–3394 (2013).
Durieux, A. C. et al. A centronuclear myopathy–dynamin 2 mutation impairs autophagy in mice. Traffic 13, 869–879 (2012).
Sarparanta, J. et al. Interactions with M-band titin and calpain 3 link myospryn (CMYA5) to tibial and limb-girdle muscular dystrophies. J. Biol. Chem. 285, 30304–30315 (2010).
McClelland, V. et al. Vici syndrome associated with sensorineural hearing loss and evidence of neuromuscular involvement on muscle biopsy. Am. J. Med. Genet. A 152A, 741–747 (2010).
Byrne, S. et al. EPG5-related Vici syndrome: a paradigm of neurodevelopmental disorders with defective autophagy. Brain 139, 765–781 (2016).
Rokach, O. et al. Epigenetic changes as a common trigger of muscle weakness in congenital myopathies. Hum. Mol. Genet. 24, 4636–4647 (2015).
North, K. N. et al. Approach to the diagnosis of congenital myopathies. Neuromuscul. Disord. 24, 97–116 (2014).
Hacohen, Y. et al. Fetal acetylcholine receptor inactivation syndrome: a myopathy due to maternal antibodies. Neurol. Neuroimmunol. Neuroinflamm. 2, e57 (2015).
Kinali, M. et al. Congenital myasthenic syndromes in childhood: diagnostic and management challenges. J. Neuroimmunol. 201–202, 6–12 (2008).
Bonnemann, C. G. et al. Diagnostic approach to the congenital muscular dystrophies. Neuromuscul. Disord. 24, 289–311 (2014).
Selcen, D. Myofibrillar myopathies. Neuromuscul. Disord. 21, 161–171 (2011).
Nishino, I. Autophagic vacuolar myopathy. Semin. Pediatr. Neurol. 13, 90–95 (2006).
Wang, C. H. et al. Consensus statement on standard of care for congenital myopathies. J. Child Neurol. 27, 363–382 (2012).
Jungbluth, H., Ochala, J., Treves, S. & Gautel, M. Current and future therapeutic approaches to the congenital myopathies. Semin. Cell Dev. Biol. 64, 191–200 (2017).
Childers, M. K. et al. Gene therapy prolongs survival and restores function in murine and canine models of myotubular myopathy. Sci. Transl Med. 6, 220ra10 (2014).
Rendu, J. et al. Exon skipping as a therapeutic strategy applied to an RYR1 mutation with pseudo-exon inclusion causing a severe core myopathy. Hum. Gene Ther. 24, 702–713 (2013).
Monnier, N. et al. A homozygous splicing mutation causing a depletion of skeletal muscle RYR1 is associated with multi-minicore disease congenital myopathy with ophthalmoplegia. Hum. Mol. Genet. 12, 1171–1178 (2003).
Barton-Davis, E. R., Cordier, L., Shoturma, D. I., Leland, S. E. & Sweeney, H. L. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J. Clin. Invest. 104, 375–381 (1999).
MacArthur, D. G. & Lek, M. The uncertain road towards genomic medicine. Trends Genet. 28, 303–305 (2012).
Cowling, B. S. et al. Reducing dynamin 2 expression rescues X-linked centronuclear myopathy. J. Clin. Invest. 124, 1350–1363 (2014).
Sabha, N. et al. PIK3C2B inhibition improves function and prolongs survival in myotubular myopathy animal models. J. Clin. Invest. 126, 3613–3625 (2016).
Ravenscroft, G. et al. Cardiac α-actin over-expression therapy in dominant ACTA1 disease. Hum. Mol. Genet. 22, 3987–3997 (2013).
Nowak, K. J. et al. Nemaline myopathy caused by absence of α-skeletal muscle actin. Ann. Neurol. 61, 175–184 (2007).
Lawlor, M. W. et al. Enzyme replacement therapy rescues weakness and improves muscle pathology in mice with X-linked myotubular myopathy. Hum. Mol. Genet. 22, 1525–1538 (2013).
Fruen, B. R., Mickelson, J. R. & Louis, C. F. Dantrolene inhibition of sarcoplasmic reticulum Ca2+ release by direct and specific action at skeletal muscle ryanodine receptors. J. Biol. Chem. 272, 26965–26971 (1997).
Timmins, M. A. et al. Malignant hyperthermia testing in probands without adverse anesthetic reaction. Anesthesiology 123, 548–556 (2015).
Michalek-Sauberer, A. & Gilly, H. Prophylactic use of dantrolene in a patient with central core disease. Anesth. Analg. 86, 915–916 (1998).
Jungbluth, H., Dowling, J. J., Ferreiro, A. & Muntoni, F. 217th ENMC International Workshop: RYR1-related myopathies, Naarden, The Netherlands, 29–31 January 2016. Neuromuscul. Disord. 26, 624–633 (2016).
Andersson, D. C. & Marks, A. R. Fixing ryanodine receptor Ca leak — a novel therapeutic strategy for contractile failure in heart and skeletal muscle. Drug Discov. Today Dis. Mech. 7, e151–e157 (2010).
Marks, A. R. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J. Clin. Invest. 123, 46–52 (2013).
Pold, R. et al. Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes 54, 928–934 (2005).
Lanner, J. T. et al. AICAR prevents heat-induced sudden death in RyR1 mutant mice independent of AMPK activation. Nat. Med. 18, 244–251 (2012).
de Winter, J. M. et al. Troponin activator augments muscle force in nemaline myopathy patients with nebulin mutations. J. Med. Genet. 50, 383–392 (2013).
de Winter, J. M. et al. Effect of levosimendan on the contractility of muscle fibers from nemaline myopathy patients with mutations in the nebulin gene. Skelet. Muscle 5, 12 (2015).
Amthor, H. & Hoogaars, W. M. Interference with myostatin/ActRIIB signaling as a therapeutic strategy for Duchenne muscular dystrophy. Curr. Gene Ther. 12, 245–259 (2012).
Durham, W. J. et al. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell 133, 53–65 (2008).
Dowling, J. J. et al. Oxidative stress and successful antioxidant treatment in models of RYR1-related myopathy. Brain 135, 1115–1127 (2012).
Natera-de Benito, D. et al. KLHL40-related nemaline myopathy with a sustained, positive response to treatment with acetylcholinesterase inhibitors. J. Neurol. 263, 517–523 (2016).
Robb, S. A. et al. Impaired neuromuscular transmission and response to acetylcholinesterase inhibitors in centronuclear myopathies. Neuromuscul. Disord. 21, 379–386 (2011).
Gibbs, E. M. et al. Neuromuscular junction abnormalities in DNM2-related centronuclear myopathy. J. Mol. Med. 91, 727–737 (2013).
Dowling, J. J. et al. Myotubular myopathy and the neuromuscular junction: a novel therapeutic approach from mouse models. Dis. Model. Mech. 5, 852–859 (2012).
Messina, S. et al. Pilot trial of salbutamol in central core and multi-minicore diseases. Neuropediatrics 35, 262–266 (2004).
Schreuder, L. T. et al. Successful use of albuterol in a patient with central core disease and mitochondrial dysfunction. J. Inherit. Metab. Dis. 33, S205–209 (2010).
Jungbluth, H., Dowling, J. J., Ferreiro, A. & Muntoni, F. 182nd ENMC International Workshop: RYR1-related myopathies, 15–17th April 2011, Naarden, The Netherlands. Neuromuscul. Disord. 22, 453–462 (2012).
Nguyen, M. A. et al. Hypertrophy and dietary tyrosine ameliorate the phenotypes of a mouse model of severe nemaline myopathy. Brain 134, 3516–3529 (2011).
Ryan, M. M. et al. Dietary L-tyrosine supplementation in nemaline myopathy. J. Child Neurol. 23, 609–613 (2008).
Winter, L. et al. Chemical chaperone ameliorates pathological protein aggregation in plectin-deficient muscle. J. Clin. Invest. 124, 1144–1157 (2014).
Kusaczuk, M., Bartoszewicz, M. & Cechowska-Pasko, M. Phenylbutyric acid: simple structure — multiple effects. Curr. Pharm. Des. 21, 2147–2166 (2015).
Cuadrado-Tejedor, M., Ricobaraza, A. L., Torrijo, R., Franco, R. & Garcia-Osta, A. Phenylbutyrate is a multifaceted drug that exerts neuroprotective effects and reverses the Alzheimer's disease-like phenotype of a commonly used mouse model. Curr. Pharm. Des. 19, 5076–5084 (2013).
Lee, C. S. et al. A chemical chaperone improves muscle function in mice with a RyR1 mutation. Nat. Commun. 8, 14659 (2017).
Vega, H., Agellon, L. B. & Michalak, M. The rise of proteostasis promoters. IUBMB Life 68, 943–954 (2016).
Yuste-Checa, P. et al. Pharmacological chaperoning: a potential treatment for PMM2-CDG. Hum. Mutat. 38, 160–168 (2017).
Kim, E., et al. Characterization of human cardiac calsequestrin and its deleterious mutants. J. Mol. Biol. 373, 1047–1057 (2007).
Tang, L., et al. Structural basis for Ca2+ selectivity of a voltage-gated calcium channel. Nature 505, 56–61 (2014).
Zamoon, J., Mascioni, A., Thomas, D. D. & Veglia, G. NMR solution structure and topological orientation of monomeric phospholamban in dodecylphosphocholine micelles. Biophys. J. 85, 2589–2598 (2003).
Efremov, R. G., Leitner, A., Aebersold, R. & Raunser, S. Architecture and conformational switch mechanism of the ryanodine receptor. Nature 517, 39–43 (2015).
Toyoshima, C., Nakasako, M., Nomura, H. & Ogawa, H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 405, 647–655 (2000).
Author information
Authors and Affiliations
Contributions
H.J., S.T., F.Z., J.O., C.S., M.G. and F.M. researched data for the article. All authors made substantial contributions to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Jungbluth, H., Treves, S., Zorzato, F. et al. Congenital myopathies: disorders of excitation–contraction coupling and muscle contraction. Nat Rev Neurol 14, 151–167 (2018). https://doi.org/10.1038/nrneurol.2017.191
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2017.191
This article is cited by
-
“RYR1 and the cerebellum”: scientific commentary on “Defective Cerebellar Ryanodine Receptor Type 1 and Endoplasmic Reticulum Calcium ‘Leak’ in Tremor Pathophysiology”
Acta Neuropathologica (2024)
-
Sporadic late onset nemaline myopathy with concurrent dermatological symptoms responding to immunosuppressive treatment
BMC Neurology (2023)
-
A review of major causative genes in congenital myopathies
Journal of Human Genetics (2023)
-
Requirement for PINCH in skeletal myoblast differentiation
Cell and Tissue Research (2023)
-
Variants in ASPH cause exertional heat illness and are associated with malignant hyperthermia susceptibility
Nature Communications (2022)